ENDURANCE-4 Study: glecaprevir/pibrentasvir in genotype 4, 5 or 6
Asselah T. Clin Gastroenterol Hepatol 2017 ; Sept 22 (Epub)
Anti-HCV
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Genotype
4
5
6
4
5
6
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
No
No
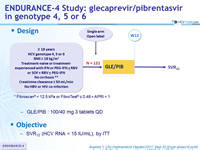
Design
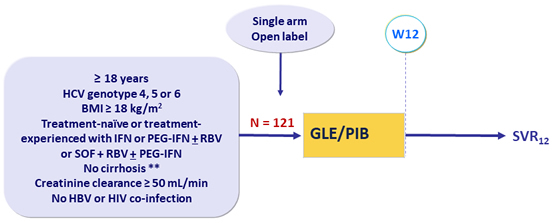
* Fibroscan® < 12.5 kPa or FibroTes® ≤ 0.48 + APRI < 1
- GLE/PIB : 100/40 mg 3 tablets QD
Objective
- SVR12 (HCV RNA < 15 IU/mL), by ITT
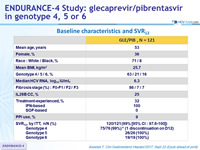
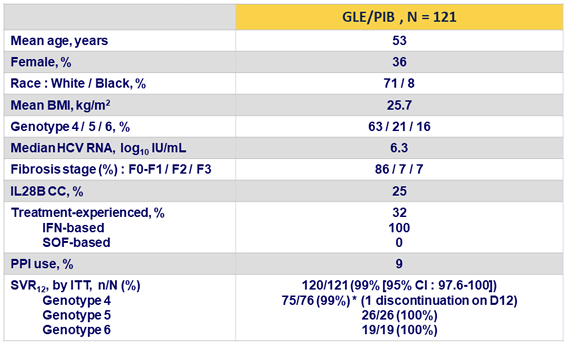
Baseline characteristics and SVR12
Adverse events and laboratory abnormalities
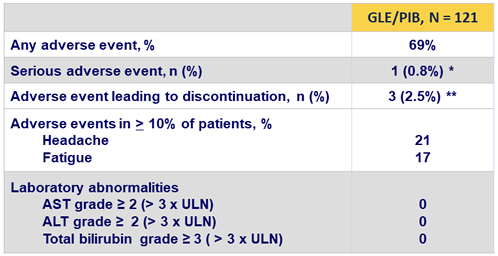
* 1 patient with baseline risk factors discontinued treatment on D12 due to a transient ischemic attack (TIA); a second TIA occurred 24 days following discontinuation. This patient has not yet returned for SVR12 visit
** 3 patients presented with anxiety, heartburn, and transient ischemic attack, respectively. Two of the 3 patients achieved SVR12
Summary
- 99% of patients with genotype 4, 5 or 6 (120/121) without cirrhosis achieved SVR12 in ITT population following treatment with 12 weeks of GLE/PIB, with no virologic failures
- 100% SVR12 in mITT population
- GLE/PIB was well tolerated
- serious adverse events occurred in < 1% of patients
- there were no grade 3 or higher laboratory abnormalities
- discontinuation due to adverse events were rare
- 12-week treatment with the IFN- and RBV-free, once-daily GLE/PIB oral regimen can successfully treat patients with HCV genotype 4, 5, or 6, regardless of prior treatment experience or F0–F3 fibrosis status