UNITY-1 Study: daclatasvir/asunaprevir/beclabuvir in genotype 1 without cirrhosis
Fixed-dose combination therapy with daclatasvir, asunaprevir and beclabuvir for non-cirrhotic patients with HCV genotype 1 infection
Poordad F. JAMA 2015;313:1728-35
Anti-HCV
Daclatasvir
Asunaprevir
Beclabuvir
Daclatasvir
Asunaprevir
Beclabuvir
Genotype
1
1a
1b
1
1a
1b
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
No
No
Design
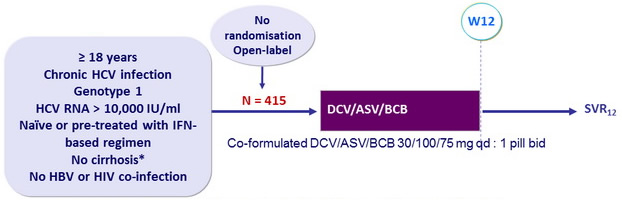
* Liver biopsy with Metavir < F4, or Fibrotest® ≤ 0.48 + APRI < 1, or Fibroscan kPa ≤ 9.6
Objective
- Primary endpoint : SVR12 (HCV RNA < 25 IU/ml) in treatment-naïve patients, non-inferiority margin of 15% = lower bound of 2-sided 95% CI > 79%, rate of historical control (SVR achieved in this population with SOF + PEG-IFN + RBV)
- Secondary endpoint : SVR12 (HCV RNA < 25 IU/ml) in treatment-experienced patients, with lower bound of 2-sided 95% CI > 49%, rate of historical control (composite SVR in this population with SMV + PEG-IFN + RBV), 95% power
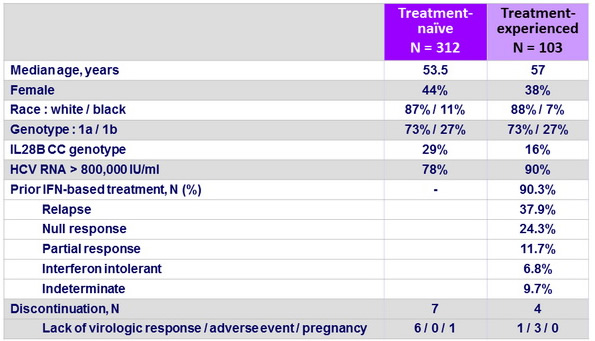
Baseline characteristics and patient disposition
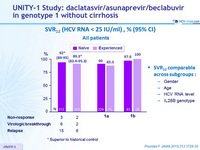
SVR12 (HCV RNA < 25 IU/ml) , % (95% CI) - All patients
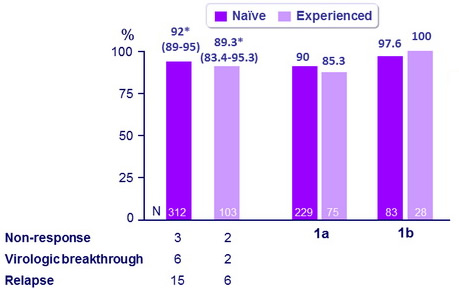
* Superior to historical control
SVR12 comparable across subgroups :
- Gender
- Age
- HCV RNA level
- IL28B genotype
Resistance analysis
- Genotype 1a : 32 virologic failures
- NS5A resistance-associated variants in 28/29 (most frequent : Q30)
- NS3 RAVs in 25/26 (most frequent : R155)
- NS5B RAVs in 12/28 (most frequent : P495)
- Genotype 1b : 2 virologic failures
- Baseline NS5A polymorphims (28, 30, 31, 93) associated with resistance to DCV
- Genotype 1a : 34/102 (11%) : SVR12 in 25/34 (74%)
- Genotype 1b : 17/106 (16%) : SVR12 in 17/17 (100%)
- Baseline NS3 and NS5B variants did not affect SVR12
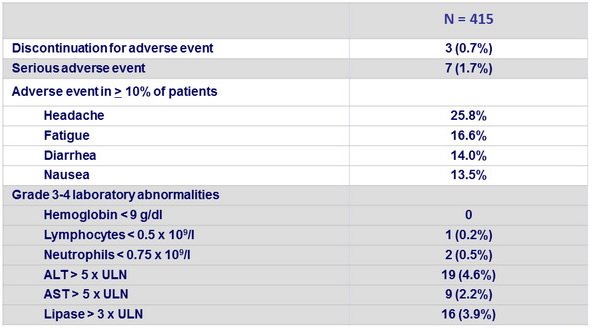
Adverse events and laboratory abnormalities, N
Summary
- In this open-label, uncontrolled study, 12 weeks of treatment with a fixed-dose combination of daclatasvir, asunaprevir and beclabuvir in HCV genotype 1-infected patients without cirrhosis was associated with high SVR12
- 92% in treatment-naive patients
- 89% in patients previously treated for HCV infection
- SVR12 rates were higher with genotype 1b than with genotype 1a in both the treatment-naive (98% vs 90%, respectively) and -experienced (100% vs 85%, respectively) cohorts
- There were low rates of serious AEs and treatment discontinuations
- All genotype 1b-infected patients with baseline NS5A polymorphisms achieved SVR12 while only 74% with genotype 1a had SVR12
- Resistance-associated variants at amino acids positions NS5A-Q30, NS3-R155K and NS5B-P495 were observed most frequently at viral breakthrough; NS5B variants were generally not observed in patients experiencing relapse