NGM282 in NASH: 3 mg QD (phase 2)
Harrison SA, EASL 2018, Abs. GS-014
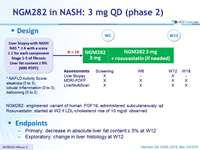
Design
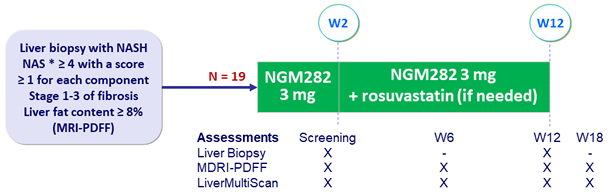
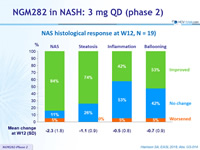
* NAFLD Activity Score: steatosis (0 to 3), lobular inflammation (0 to 3), ballooning (0 to 2)
- NGM282: engineered variant of human FGF19, administered subcutaneously qd
- Rosuvastatin: started at W2 if LDL-cholesterol rise of 10 mg/dl observed
Endpoints
- Primary: decrease in absolute liver fat content = 5% at W12
- Exploratory: change in liver histology at W12
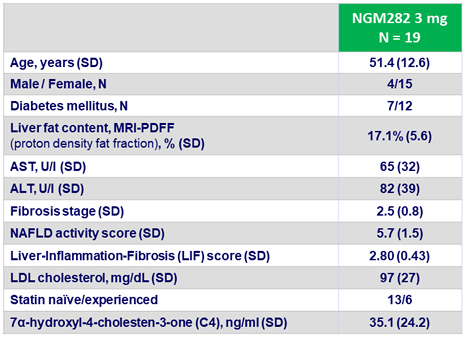
Baseline characteristics, mean or N or %
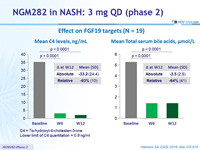
Effect on FGF19 targets (N = 19)
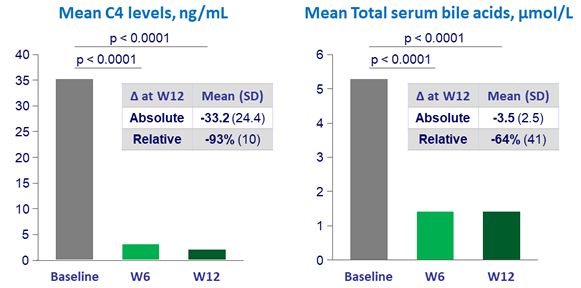
C4 = 7a-hydroxyl-4-cholesten-3-one
Lower limit of C4 quantitation = 0.9 ng/ml
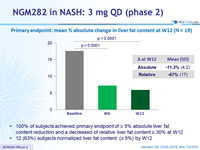
Primary endpoint: mean % absolute change in liver fat content at W12 (N = 19)
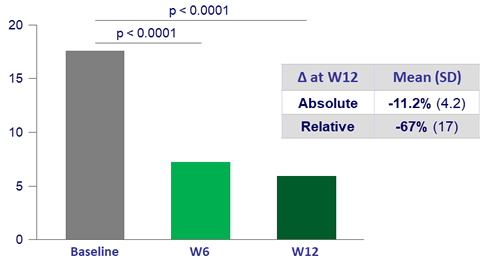
- 100% of subjects achieved primary endpoint of = 5% absolute liver fat content reduction and a decreased of relative liver fat content = 30% at W12
- 12 (63%) subjects normalized liver fat content (= 5%) by W12
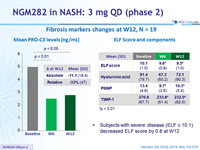
Fibrosis markers changes at W12, N = 19
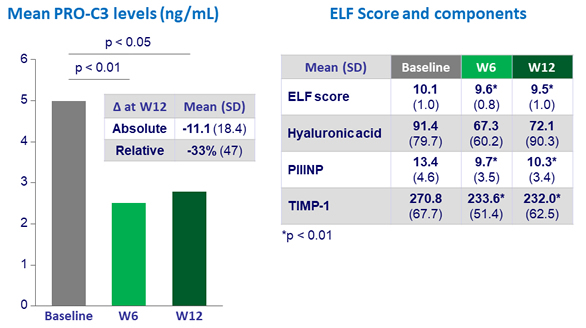
- Subjects with severe disease (ELF = 10.1) decreased ELF score by 0.8 at W12
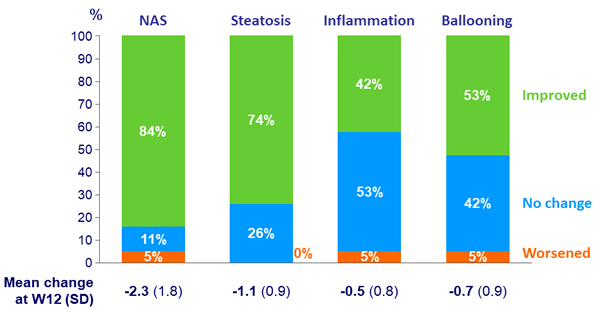
NAS histological response at W12, N = 19
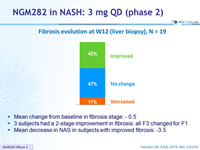
Fibrosis evolution at W12 (liver biopsy), N = 19
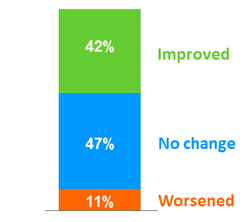
- Mean change from baseline in fibrosis stage: - 0.5
- 3 subjects had a 2-stage improvement in fibrosis: all F3 changed for F1
- Mean decrease in NAS in subjects with improved fibrosis: -3.5
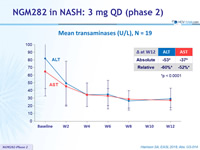
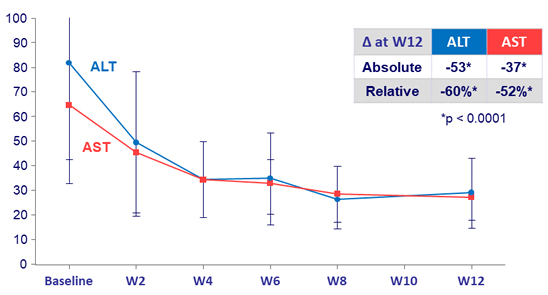
Mean transaminases (U/L), N = 19
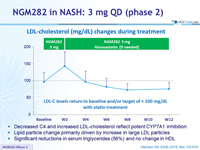
LDL-cholesterol (mg/dL) changes during treatment
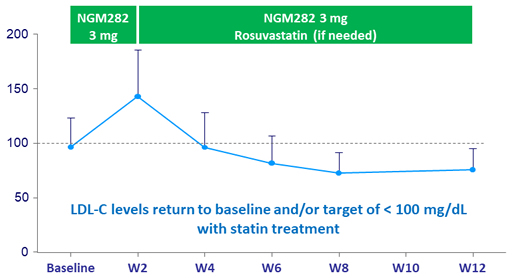
- Decreased C4 and increased LDL-cholesterol reflect potent CYP7A1 inhibition
- Lipid particle change primarily driven by increase in large LDL particles
- Significant reductions in serum triglycerides (56%) and no change in HDL
Safety and tolerability
- Favorable safety and tolerability profile consistent with other NGM282 studies (no new safety signals identified)
- Mild gastrointestinal symptoms (loose/frequent stools) remain the most common treatment emergent adverse events
- Majority were mild and resolved during treatment phase
- No subject withdrew from treatment due to any drug-related adverse events
- Gastrointestinal symptoms were largely mitigated with separating the timing of injection around meals and decreasing meal size
- Three severe adverse events, all unrelated to study drug:
- Pleurisy
- Chest tightness
- Cardiac arrest (non myocardial infarction)
Summary
- Potent C4 and bile acid suppression consistent with FGF19 hormone activity
- Significant and clinically meaningful reductions across non-invasive markers of NASH-related disease
- Large percentage of patients demonstrated histological improvement at W12
- Statin co-administration rapidly mitigates LDL-cholesterol elevations
- Treatment was safe and well tolerated