Selonsertib in NASH: phase 2
Loomba R, Hepatology 2018;67:549-59
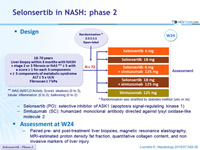
Design
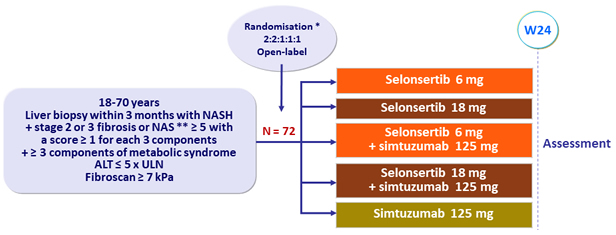
* Randomisation was stratified by diabetes mellitus (yes or no)
** NAS ( NAFLD Activity Score): steatosis (0 to 3), lobular inflammation (0 to 3), ballooning (0 to 2)
- Selonsertib (PO): selective inhibitor of ASK1 (apoptosis signal-regulating kinase 1)
- Simtuzumab (SC): humanized monoclonal antibody directed against lysyl oxidase-like molecule 2
Assessment at W24
- Paired pre- and post-treatment liver biopsies, magnetic resonance elastography, MRI-estimated proton density fat fraction, quantitative collagen content, and non invasive markers of liver injury
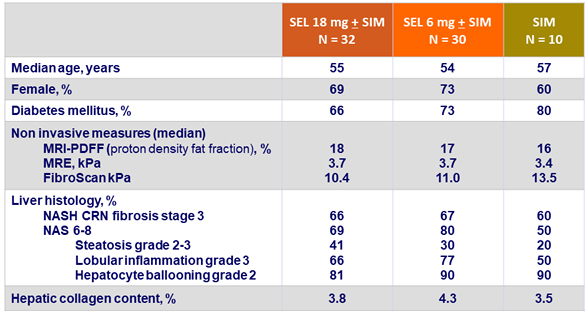
Baseline characteristics
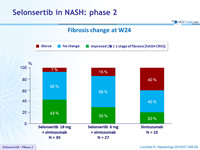
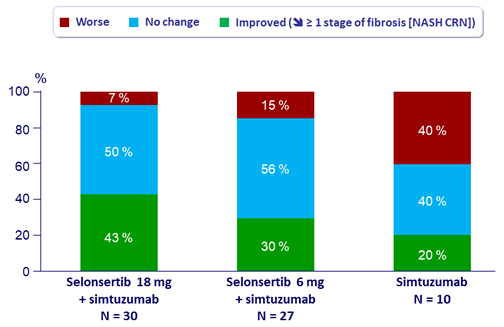
Fibrosis change at W24
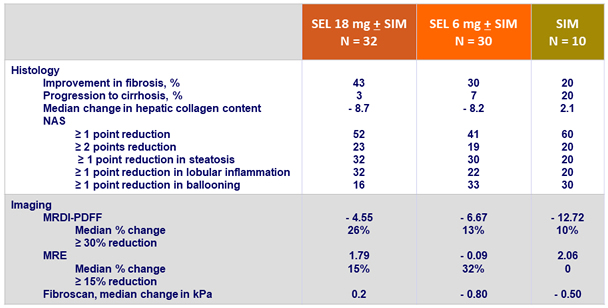
Changes in histology and imaging at W24
Factors associated with fibrosis improvement
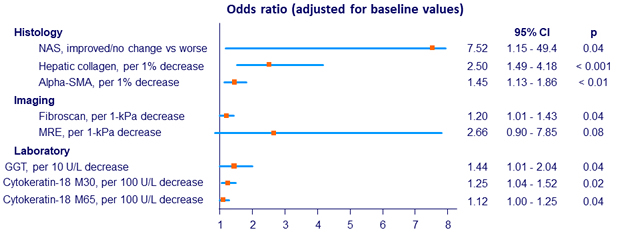
SMA: smooth muscle actin ; MRE: magnetic resonance elastography
- Changes in body weight, MRI-PDFF and grades of steatosis and ballooning were not associated with fibrosis improvement
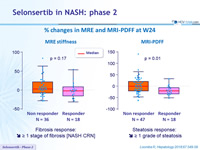
% changes in MRE and MRI-PDFF at W24
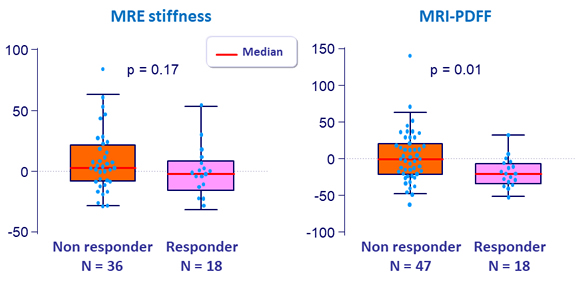
- Fibrosis response: dec. = 1 stage of fibrosis [NASH CRN]
- Steatosis response: dec. = 1 grade of steatosis
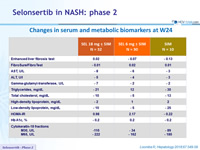
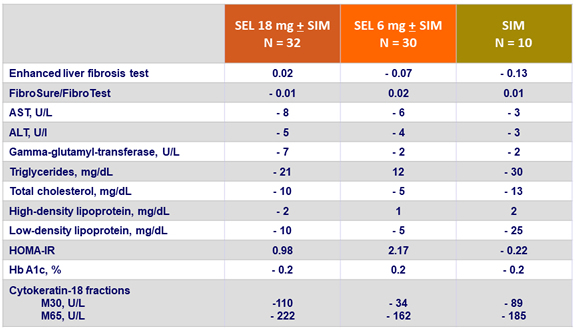
Changes in serum and metabolic biomarkers at W24
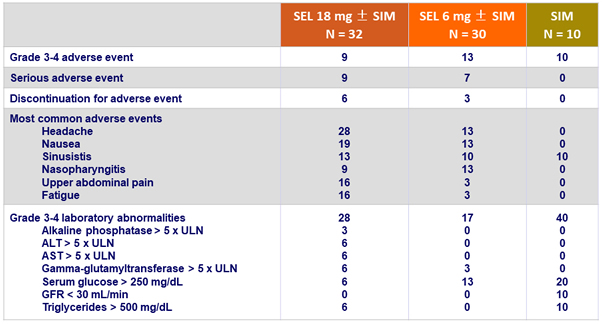
Adverse events and laboratory abnormalities, %
Summary
- In this phase 2 exploratory trial, selonsertib appeared to improve liver fibrosis in a substantial proportion of patients with NASH and stage 2 or 3 fibrosis
- This suggests that selonsertib has the potential to help address an important unmet medical need for an effective antifibrotic therapy for patients with NASH and advanced fibrosis
- Rationale for phase 3 studies of selonsertib in patients with NASH and bridging fibrosis (STELLAR-3) and compensated cirrhosis (STELLAR-4)