Study 337-1119: LDV/SOF in genotype 5
Abergel A. Lancet Infect Dis. 2016;16:459-64
Anti-HCV
Ledipasvir
Sofosbuvir
Ledipasvir
Sofosbuvir
Genotype
5
5
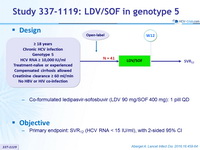
Design
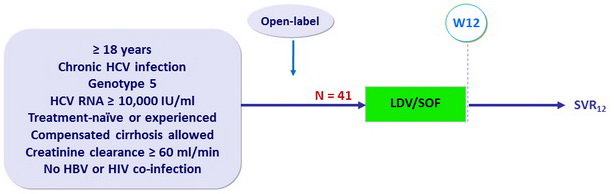
- Co-formulated ledipasvir-sofosbuvir (LDV 90 mg/SOF 400 mg): 1 pill QD
Objective
- Primary endpoint: SVR12 (HCV RNA < 15 IU/ml), with 2-sided 95% CI
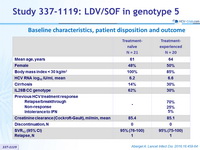
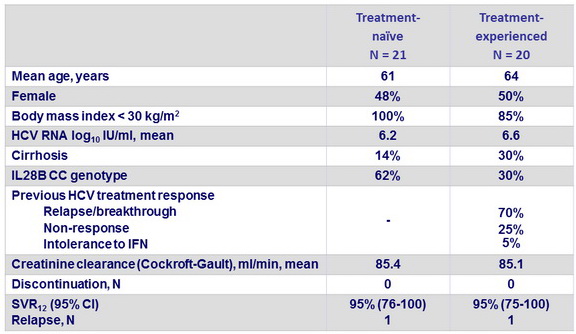
Baseline characteristics, patient disposition and outcome
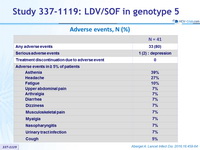
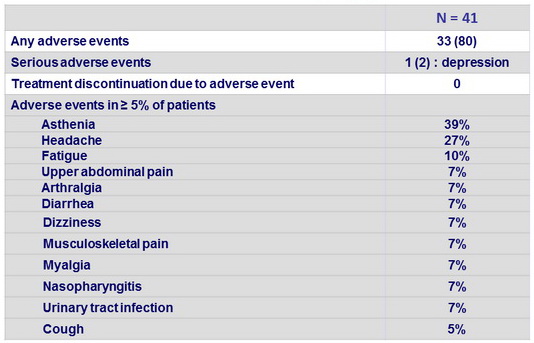
Adverse events, N (%)
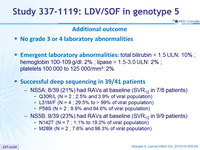
Additional outcome
- No grade 3 or 4 laboratory abnormalities
- Emergent laboratory abnormalities : total bilirubin < 1.5 ULN: 10% ; hemoglobin 100-109 g/dl: 2% ; lipase > 1.5-3.0 ULN: 2% ; platelets 100 000 to 125 000/mm3 : 2%
- Successful deep sequencing in 39/41 patients
- NS5A: 8/39 (21%) had RAVs at baseline (SVR12 in 7/8 patients)
- Q30R /L (N = 2 ; 2.5 % and 3.9% of viral population)
- L31M /F (N = 4 ; 29.5% to > 99 % of viral population)
- P58S (N = 2 ; 9.9% and 94.6% of viral population)
- NS5B: 9/39 (23%) had RAVs at baseline (SVR12 in 9/9 patients)
- N142T (N = 7 ; 1.1 % to 19.2% of viral population)
- M289I (N = 2 ; 7.6% and 98.3% of viral population )
- NS5A: 8/39 (21%) had RAVs at baseline (SVR12 in 7/8 patients)
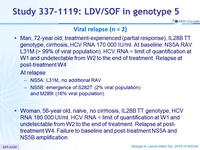
Viral relapse (n = 2)
- Man, 72-year old, treatment-experienced (partial response), IL28B TT genotype, cirrhosis, HCV RNA 170 000 IU/ml. At baseline: NS5A RAV L31M (> 99% of viral population). HCV RNA < limit of quantification at W1 and undetectable from W2 to the end of treatment. Relapse at post-treatment W4
At relapse- NS5A: L31M, no additional RAV
- NS5B: emergence of S282T (2% viral population)
and M289I (16% viral population)
- Woman, 56-year old, naïve, no cirrhosis, IL28B TT genotype, HCV RNA 180 000 UI/ml. HCV RNA < limit of quantification at W1 and undetectable from W2 to the end of treatment. Relapse at post-treatment W4. Failure to baseline and post-treatment NS5A and NS5B amplification
Summary
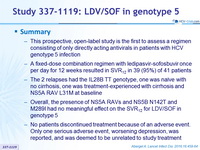
- This prospective, open-label study is the first to assess a regimen consisting of only directly acting antivirals in patients with HCV genotype 5 infection
- A fixed-dose combination regimen with ledipasvir-sofosbuvir once per day for 12 weeks resulted in SVR12 in 39 (95%) of 41 patients
- The 2 relapses had the IL28B TT genotype, one was naïve with no cirrhosis, one was treatment-experienced with cirrhosis and NS5A RAV L31M at baseline
- Overall, the presence of NS5A RAVs and NS5B N142T and M289I had no meaningful effect on the SVR12 for LDV/SOF in genotype 5
- No patients discontinued treatment because of an adverse event. Only one serious adverse event, worsening depression, was reported, and was deemed to be unrelated to study treatment