GS-US-367-1169 Study: SOF/VEL + GS-9857
in genotype 2, 3, 4 or 6 - Phase II
Gane EJ, Gastroenterology 2016; 151:902-909
Anti-HCV
Voxilaprevir (GS-9857)
Velpatasvir (GS-5816)
Sofosbuvir
Voxilaprevir (GS-9857)
Velpatasvir (GS-5816)
Sofosbuvir
Genotype
2
3
4
2
3
4
Treatment history
Naive
IFN-Experienced
SOF-experienced
Naive
IFN-Experienced
SOF-experienced
Cirrhosis
Yes
No
Yes
No
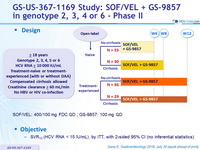
Design
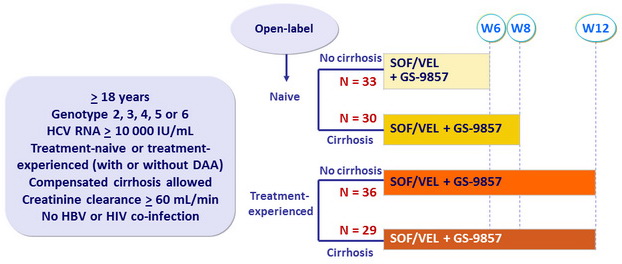
- SOF/VEL: 400/100 mg FDC QD ; GS-9857: 100 mg QD
Objective
- SVR12 (HCV RNA < 15 IU/mL) , by ITT, with 2-sided 95% CI (no inferential statistics)
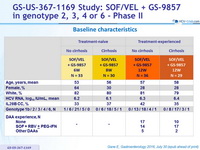
Baseline characteristics
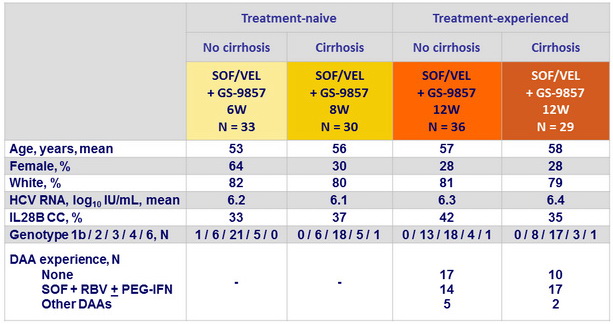
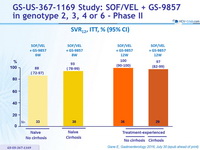
SRV12, ITT, % (95% CI)
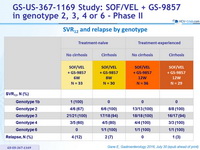
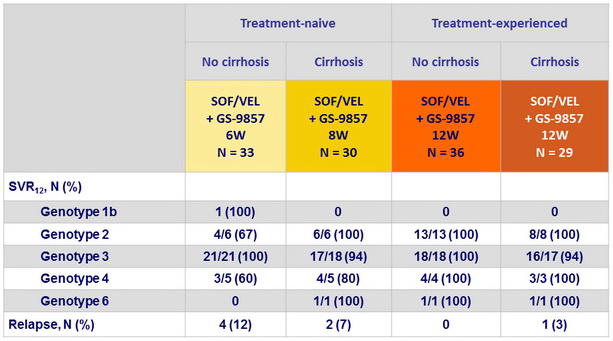
SRV12 and relapse by genotype
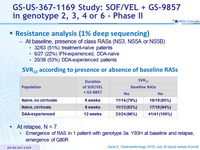
Resistance analysis (1% deep sequencing)
- At baseline, presence of class RASs (NS3, NS5A or NS5B)
- 32/63 (51%) treatment-naïve patients
- 6/27 (22%) IFN-experienced, DDA-naïve
- 20/38 (53%) DDA-experienced patients
SRV12 according to presence or absence of baseline RASs
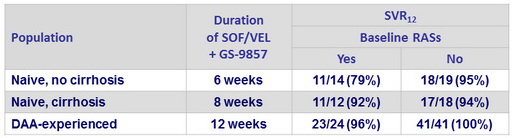
- At relapse, N = 7
- Emergence of RAS in 1 patient with genotype 3a: Y93H at baseline and relapse, emergence of Q80R
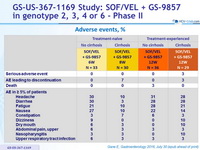
Adverse events, %
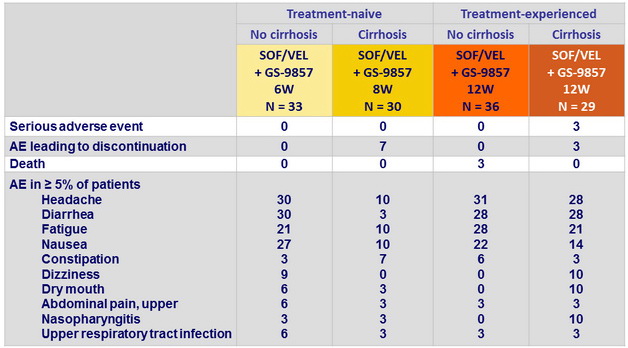
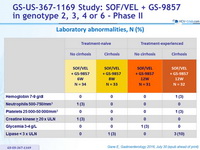
Laboratory abnormalities, N (%)
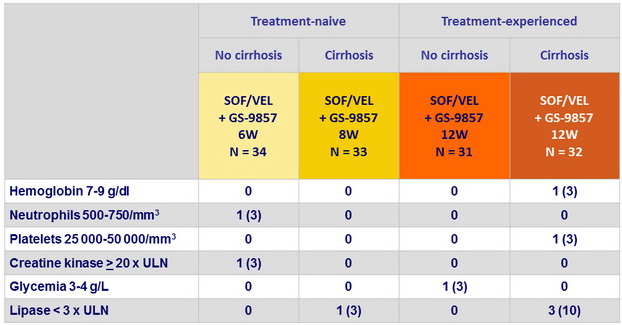
Summary
- In this phase 2 open-label trial, SOF/VEL + GS-9857 (8 weeks in treatment-naïve patients or 12 weeks in treatment-experienced patients) was safe and effective for patients with HCV genotype 2, 3, 4, or 6 infections, with or without compensated cirrhosis