PLUTO Study: SMV + SOF in genotype 4
Buti M, Aliment Pharmacol Ther 2017;45:468-75
Anti-HCV
Simeprevir
Sofosbuvir
Simeprevir
Sofosbuvir
Genotype
4
4
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
No
No
Design
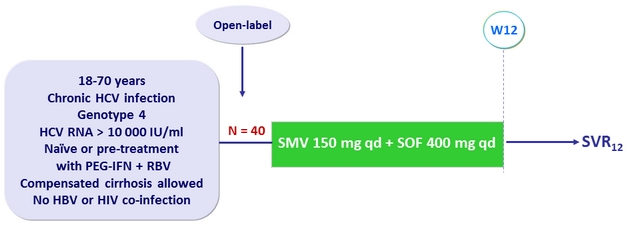
Objective
- SVR12 (HCV RNA < 15 IU/ml), with 95% CI, by ITT
- Superiority if lower limit of the 95% CI > SVR12 rate of 61% of a historical control (SMV + PEG-IFN + RBV) from a composite endpoint in RESTORE study
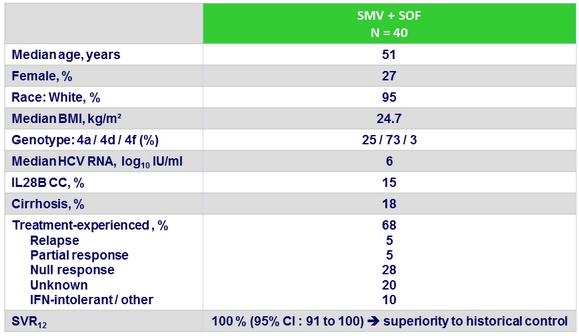
Baseline characteristics
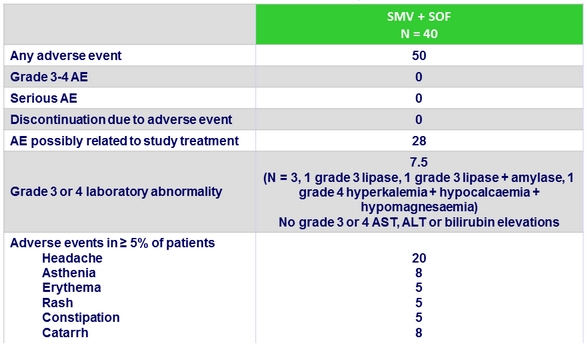
Adverse events, %
Summary
- SMV + SOF for 12 weeks resulted in overall 100% SVR12 in genotype 4 HCV infected patients
- With or without cirrhosis
- Whether naïve or IFN-PEG + RBV pretreated
- Treatment was safe and well tolerated, all adverse events being of grade 1 or 2