C-CREST study, Part B: uprifosbuvir (MK-3682)/GZR/ruzasvir (MK-8408) fixed-dose combination ± RBV for genotypes 1, 2 and 3 - Phase II
Lawitz E. Lancet Gastroenterol Hepatol 2017 ; 2 :814-823
Grazoprevir
Ruzasvir (MK-8408)
Uprifosbuvir (MK-3682)
Ribavirin
1
2
3
Naive
IFN-Experienced
Yes
No
Design
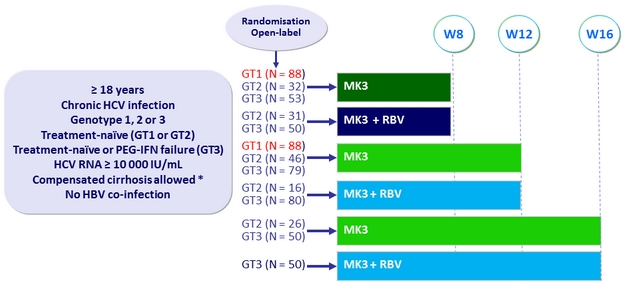
* Liver biopsy or Fibroscan® > 12.5 kPa or Fibrotest® ≥ 0.75 + APRI > 2
- Uprifosbuvir 225 mg/GZR 50 mg/ ruzasvir 30 mg FDC (MK3) = 2 tablets QD
Objective
- Primary endpoint: SVR12 ( HCV RNA < 15 IU/mL), full analysis set (patients having received ≥ 1 dose of study drug)
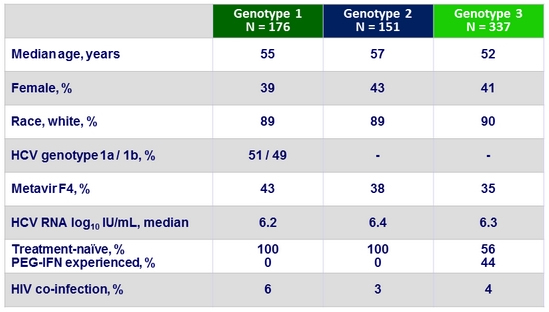
Baseline characteristics
SVR12 ( Full Analysis Set)
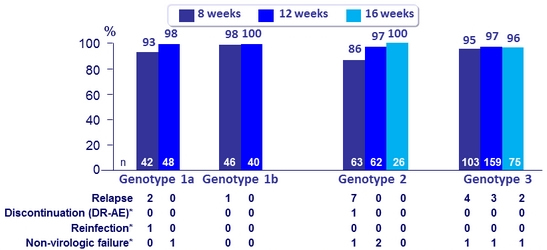
* Genotype 1a 8W, No RBV: 1 patient achieved SVR 8 but was reinfected with a different HCV strain at FW12
Genotype 1a 12W, No RBV: 1 patient died due to study-drug unrelated bacterial sepsis
Genotype 2 8W + RBV: 1 patient discontinued at D5 due to drug-related AEs of fatigue, malaise; 1 patient lost to FU
Genotype 2 12W, No RBV: 2 patients lost to follow- up
Genotype 3 8W + RBV: 1 patient lost to follow- up
Genotype 3 12W, No RBV: 1 patient withdrew due to pregnancy, lost to follow- up
Genotype 3 16W: 1 patient lost to follow-up
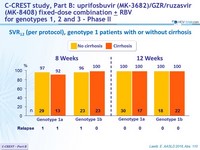
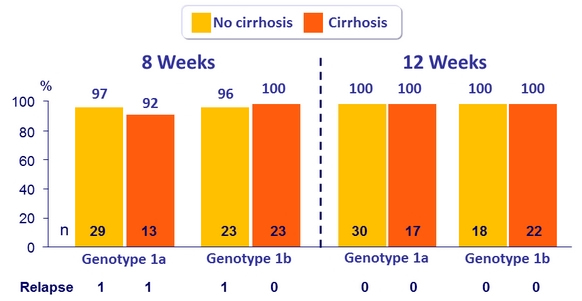
SVR12 (per protocol), genotype 1 patients with or without cirrhosis
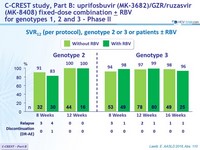
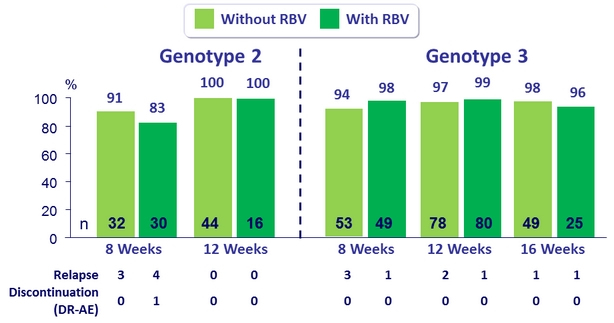
SVR12 (per protocol), genotype 2 or 3 or patients ± RBV
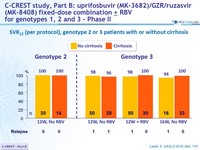
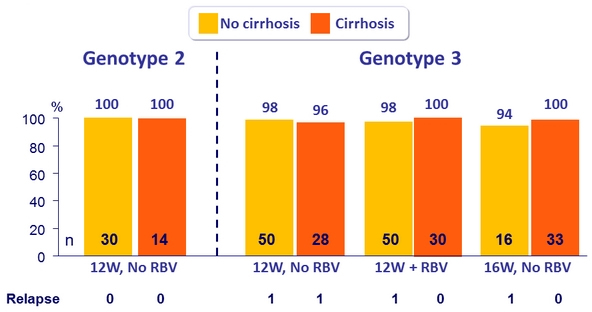
SVR12 (per protocol), genotype 2 or 3 patients with or without cirrhosis
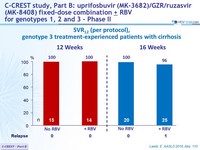
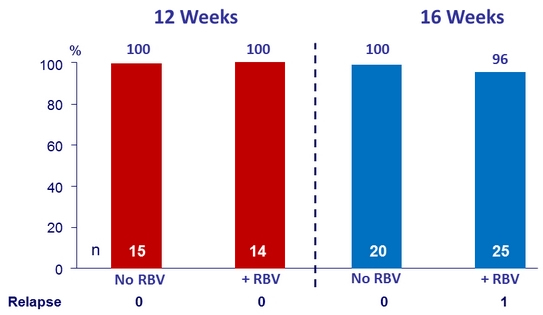
SVR12 (per protocol), genotype 3 treatment-experienced patients with cirrhosis
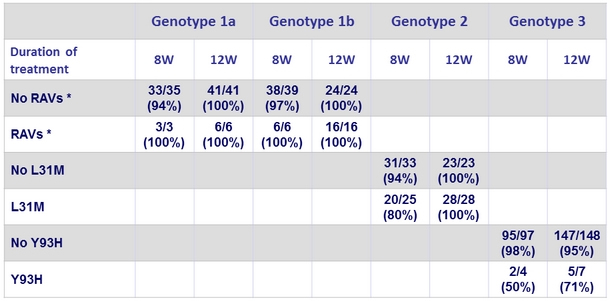
SVR12 according to the presence of NS5A RAVs
Adverse events
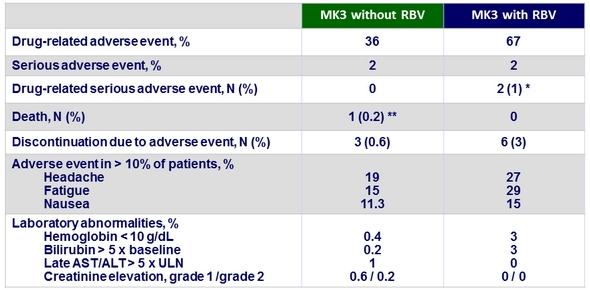
* 1 genotype 3-infected patient had an exacerbation of chronic obstructive pulmonary disease related to RBV ; 1 genotype 2-infected patient had a worsening of depression related to RBV
** 1 genotype 1-infected patient died due to a study drug-unrelated bacterial sepsis
Summary
- MK3 (uprifosbuvir / grazoprevir / ruzasvir ) for 8 or 12 weeks was highly effective in genotype 1 patients (SVR12 = 97%)
- MK3 for 12 or 16 weeks was highly effective in genotype 2 patients (SVR 12 = 98%)
- The addition of RBV did not increase SVR12
- MK3 for 8, 12 or 16 weeks was highly effective in genotype 3 treatment-naïve or treatment-experienced patients (SVR12 = 96%)
- The addition of RBV did not increase SVR12
- Efficacy was maintained in genotype 3 treatment-experienced patients with cirrhosis (SVR12 = 99%)
- Treatment with MK3 was generally safe and well-tolerated