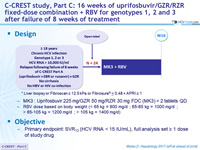
C-CREST study, Part C: 16 weeks of uprifosbuvir/GZR/RZR fixed-dose combination + RBV for genotypes 1, 2 and 3 after failure of 8 weeks of treatment
Wyles D. Hepatology 2017 ; 66 :1794-1804
Anti-HCV
Grazoprevir
Ruzasvir (MK-8408)
Uprifosbuvir (MK-3682)
Grazoprevir
Ruzasvir (MK-8408)
Uprifosbuvir (MK-3682)
Genotype
1
2
3
1
2
3
Treatment history
PI (NS3)-experienced
NS5A experienced
PI (NS3)-experienced
NS5A experienced
Cirrhosis
No
No
Design
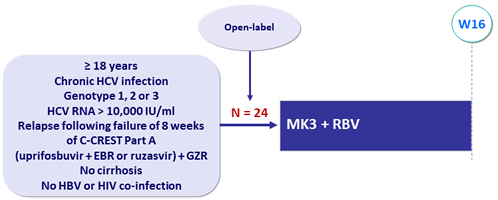
* Liver biopsy or Fibroscan ≤ 12.5 kPa or Fibrosure® ≤ 0.48 + APRI ≤ 1
- MK3 : Uprifosbuvir 225 mg/GZR 50 mg/RZR 30 mg FDC (MK3) = 2 tablets QD
- RBV dose based on body weight (< 65 kg = 800 mg/d ; 65-85 kg = 1000 mg/d ;> 85-105 kg = 1200 mg/d ; > 105 kg = 1400 mg/d )
Objective
- Primary endpoint: SVR12 (HCV RNA < 15 IU/mL), full analysis set ≥ 1 dose of study drug
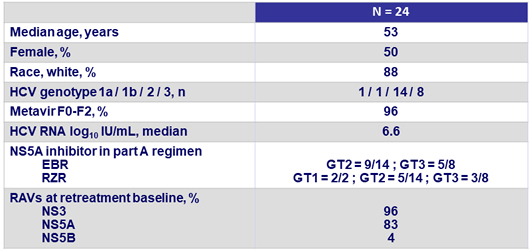
Baseline characteristics (N = 24)
SVR12, full analysis set, %

Adverse events (N = 24)
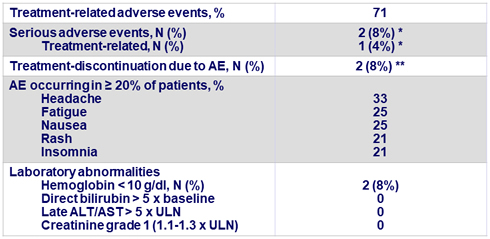
* 2 subjects had 3 SAEs:
-1 genotype 2-infected patient withdrew after a single dose with SAEs of vomiting
and tachycardia considered related to MK3 + RBV
-1 genotype 3-infected patient was hospitalized for severe anxiety, unrelated to MK3 + RBV
** 1 genotype 2-infected patient withdrew after a single dose with SAEs as above ;
1 genotype 2-infected patient discontinued RBV 4 days before the completion of 16 weeks of therapy due to rash considered RBV-related, but completed 16 weeks of MK3
Summary
- MK3 (uprifosbuvir / grazoprevir / ruzasvir) plus RBV for 16 weeks was highly effective in genotype 1, 2, and 3-infected patients without cirrhosis who had previously failed 8 weeks of treatment with a regimen of uprifosbuvir + EBR or RZR + GZR
- 100% SVR12 in 23 patients who completed treatment
- High efficacy despite a high prevalence of baseline NS3 and NS5A RAVs in this DAA failure population
- Treatment was generally safe and well-tolerated