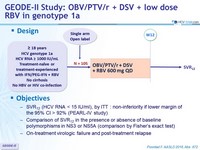
GEODE-II Study: OBV/PTV/r + DSV + low dose RBV in genotype 1a
Poordad F. AASLD 2016, Abs. 872
Anti-HCV
Paritaprevir/ritonavir
Ombitasvir
Dasabuvir
Ribavirin
Paritaprevir/ritonavir
Ombitasvir
Dasabuvir
Ribavirin
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
No
No
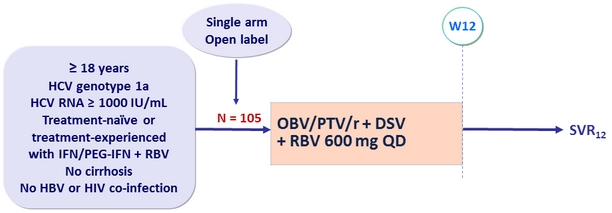
Design
Objective
- SVR12 (HCV RNA < 15 IU/ml), by ITT : non-inferiority if lower margin of the 95% CI > 92% (PEARL-IV study)
- Comparison of SVR12 in the presence or absence of baseline polymorphisms in NS3 or NS5A (comparison by Fisher's exact test)
- On-treatment virologic failure and post-treatment relapse
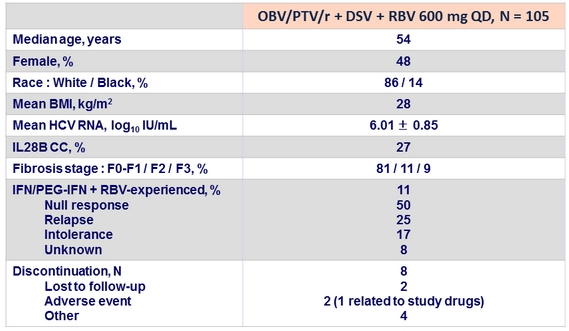
Baseline characteristics and disposition of patients
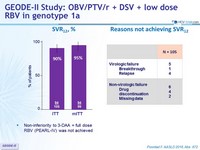
SVR12 , %
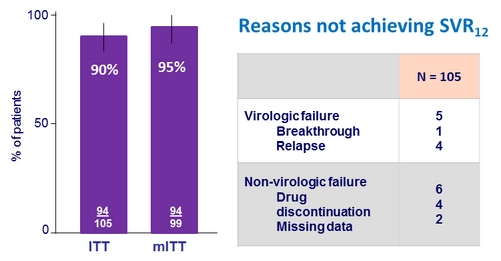
- Non-inferiority to 3-DAA + full dose RBV (PEARL-IV) was not achieved
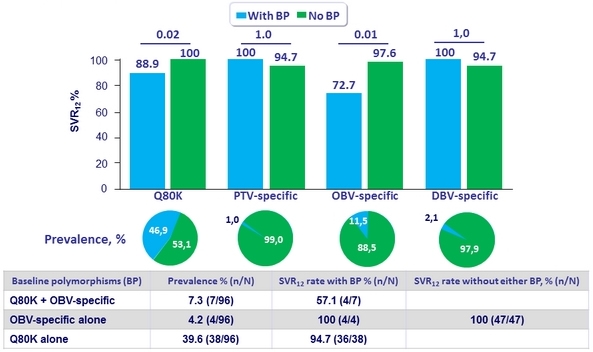
Resistance analysis : polymorphisms (alone or as components of double variants) that confer = 5-fold increase in EC50 were considered
- PTV specific RAVs: F43L, Y56H, R155G/K/S/T/W, A156S/T/V, or D168A/E/F/H/N/V/Y. Q80K (3-fold increase in EC 50 ) was evaluated separately based on the significance of this polymorphism for simeprevir (SMV)
- OBV specific RAVs: M28T/V, Q30E/K/R/Y, L31V, P32L, H58D, or Y93C/F/H/L/N/S
- DSV specific RAVs: L314H, C316Y, M414I/T/V, E446K/Q, Y448C/H, C451R, A553T, G554S, Y555
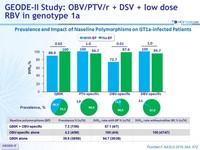
Prevalence and Impact of Naseline Polymorphisms on GT1a-infected Patients
Adverse events and laboratory abnormalities, %
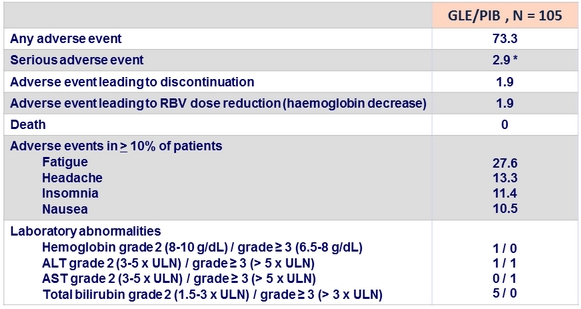
* 1 possibly related to study drug (bipolar disorder), the 2 others not related to study drugs
** 1 possibly related to study drug (chest pain, heart rate increased, and dyspnea)
Summary
- OBV/PTV/r + DSV + 600 mg RBV QD achieved a SVR12 of 90% in patients with genotype 1a in the ITT analysis and 95% in the mITT analysis
- Non-inferiority was not established for the primary study end point
- The regimen was well tolerated with mostly mild or moderate AEs. Laboratory abnormalities were lower compared to historical controls:
- Grade = 2 hemoglobin occurred in 1% of patients (compares favorably to 6.2% in historical controls who received full dose RBV)
- No Grade = 3 total bilirubin occurred (compares favorably to 5.7% in historical controls who received full dose RBV)
- Patients with baseline polymorphisms showed numerically lower SVR12
- Several patients failed to achieve SVR12 due to loss to follow-up, incarceration, and other underlying behavioral disorders (high rate of history of IV drug abuse, alcohol abuse, and cognitive or psychiatric disorders in the study population)