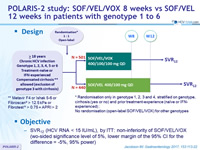
POLARIS-2 study: SOF/VEL/VOX 8 weeks vs SOF/VEL 12 weeks in patients with genotype 1 to 6
Jacobson IM. Gastroenterology 2017; 153:113-22
Anti-HCV
Voxilaprevir (GS-9857)
Velpatasvir (GS-5816)
Sofosbuvir
Voxilaprevir (GS-9857)
Velpatasvir (GS-5816)
Sofosbuvir
Genotype
1a
1b
3
4
1a
1b
3
4
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
Design
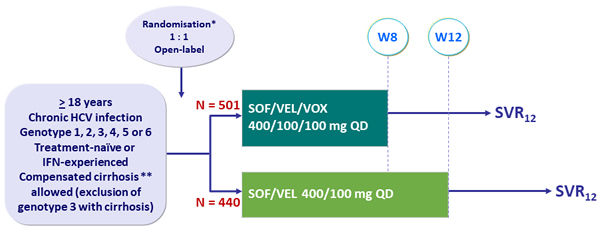
* Randomisation only in genotype 1, 2, 3 and 4, stratified on genotype, cirrhosis (yes or no) and prior treatment-experience (naïve or IFN-experienced) ; No randomisation (open-label SOF/VEL/VOX) for other genotypes
** Metavir F4 or Ishak 5-6 or Fibroscan ® > 12.5 kPa or Fibrotest ® > 0.75 + APRI > 2
Objective
- SVR12 (HCV RNA < 15 IU/mL), by ITT: non-inferiority of SOF/VEL/VOX (wo-sided significance level of 5%, lower margin of the 95% CI for the difference = - 5 %, 95% power)
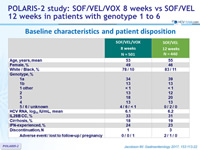
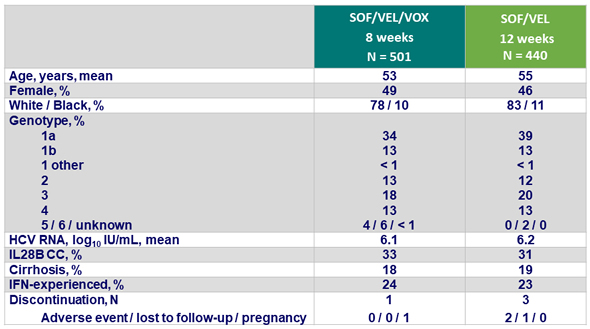
Baseline characteristics and patient disposition
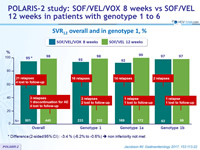
SVR12 overall and in genotype 1, %
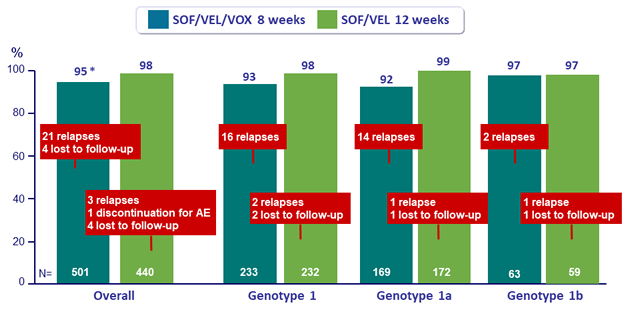
* Difference (2-sided 95% CI) : -3.4 % (-6.2% to -0.6%) » non inferiority not met
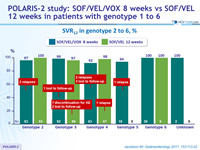
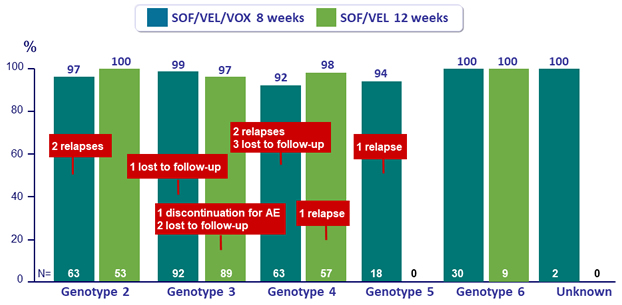
SVR12 in genotype 2 to 6, %
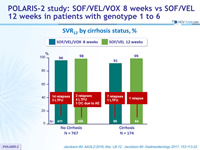
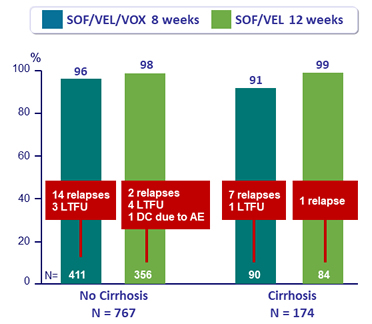
SVR12 by cirrhosis status, %
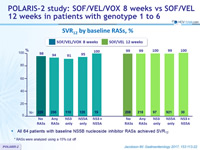
SVR12 by baseline RASs, %
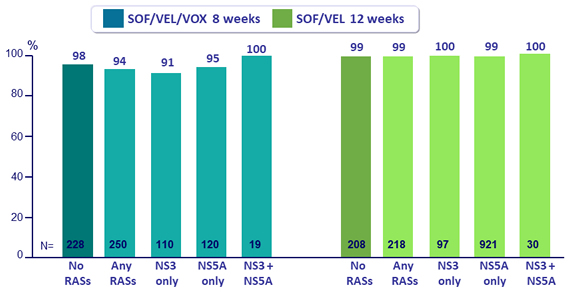
* RASs were analyzed using a 15% cut off
- All 64 patients with baseline NS5B nucleoside inhibitor RASs achieved SVR12
Impact of baseline NS5A RAS
- For patients with genotype 1a, SVR12 with SOF /VEL/ VOX 8 weeks
- 89% if baseline RASs
- 95% if no baseline RASs
- 88% if baseline Q80K
- 94% if absence of baseline 80K
Viral resistance testing at failure
- Of the 21 patients who relapsed in the SOF/VEL/VOX group, 1 had treatment-emergent NS5A resistance-associated substitutions Q30R and L31M
- Among patients receiving SOF/VEL, 1 of the 3 patients who relapsed had the Y93N variant, which is associated with resistance to NS5A inhibitors, at relapse
Adverse events
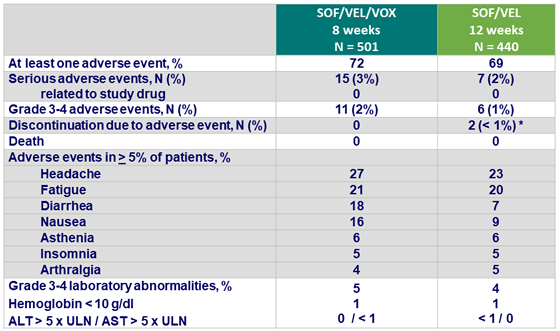
*1 upper respiratory tract infection and 1 Clostridium difficile infection, both not related to the study medication
Summary
- SOF/VEL/VOX for 8 weeks resulted in a 95% SVR12 rate in DAA-naïve genotype 1-6 patients with and without cirrhosis
- Did not meet non-inferiority as compared to the 98% SVR12 rate with SOF/VEL for 12 weeks
- The difference between the regimens was largely attributed to more relapses among patients with genotype 1a infection in the SOF/VEL/VOX group
- SOF/VEL/VOX and SOF/VEL were safe and well tolerated
- Mild gastrointestinal adverse events (nausea and diarrhea) were associated with treatment regimens that included VOX
- There was no evidence of VOX-related hepatotoxicity
- These findings in DAA-naïve patients with or without cirrhosis underscore the very high rates of SVR conferred by SOF/VEL across all HCV genotypes
- SOF/VEL/VOX for 8 weeks provides a highly efficacious and well-tolerated short-duration regimen in patients for whom adherence to a longer duration regimen may be challenging