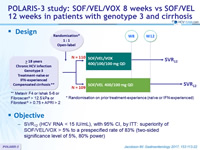
POLARIS-3 study: SOF/VEL/VOX 8 weeks vs SOF/VEL 12 weeks in patients with genotype 3 and cirrhosis
Jacobson IM. Gastroenterology 2017, 153:113-22 & Foster GR. AASLD 2016, Abs. 258
Anti-HCV
Voxilaprevir (GS-9857)
Velpatasvir (GS-5816)
Sofosbuvir
Voxilaprevir (GS-9857)
Velpatasvir (GS-5816)
Sofosbuvir
Genotype
3
3
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
Yes
Design
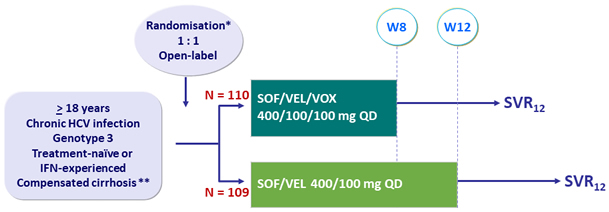
* Randomisation on prior treatment-experience (naïve or IFN-experienced)
** Metavir F4 or Ishak 5-6 or Fibroscan® > 12.5 kPa or Fibrotest® > 0.75 + APRI > 2
Objective
- SVR12 (HCV RNA < 15 IU/ml), with 95% CI, by ITT: superiority of SOF/VEL/VOX > 5% to a prespecified rate of 83% (two-sided significance level of 5%, 80% power)
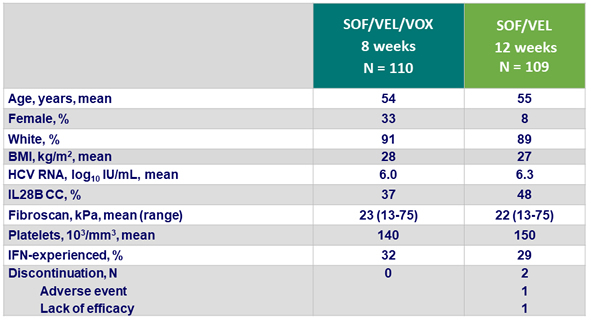
Baseline characteristics and patient disposition
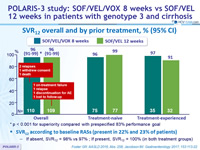
SVR12 overall and by prior treatment, % (95% CI)
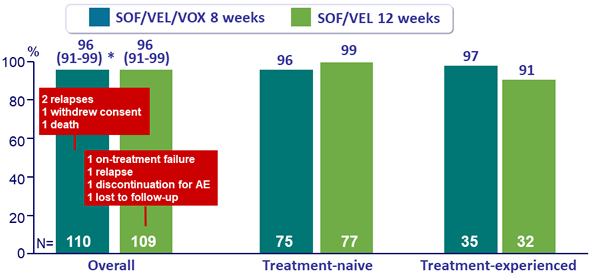
* p < 0.001 for superiority compared with prespecified 83% performance goal
SVR12 according to baseline RASs (present in 22% and 23% of patients)
- If absent, SVR12 = 98% vs 97% ; If present, SVR12 = 100% (in both treatment groups)
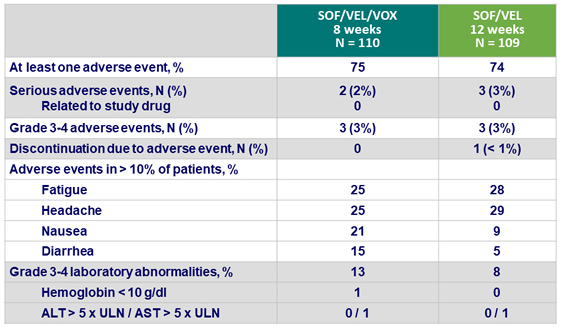
Adverse events
Summary
- In patients with HCV genotype 3 infection and cirrhosis, SOF /VEL/VOX for 8 weeks resulted in a 96% SVR12 rate as did SOF/VEL for 12 weeks
- Baseline NS5A RASs, including Y93H, did not impact treatment outcome for either SOF/VEL/VOX or SOF/VEL
- No treatment emergent RASs in the SOF/VEL/VOX group
- In the SOF/VEL group, both virologic failures had Y93H emergence
- SOF/VEL/VOX and SOF/VEL were safe and well tolerated
- Mild gastrointestinal adverse events (nausea and diarrhea) were associated with treatment regimens that included VOX
- There was no evidence of VOX-related hepatotoxicity
- SOF/VEL/VOX for 8 weeks and SOF/VEL for 12 weeks both provide simple, safe, and effective treatment regimens for patients with HCV genotype 3 and cirrhosis