POLARIS-4 study: SOF/VEL/VOX vs SOF/VEL in genotypes 1 to 6 with non-NS5A inhibitor experience
Bourlière M. NEJM 2017; 376:2134-46
Anti-HCV
Voxilaprevir (GS-9857)
Velpatasvir (GS-5816)
Sofosbuvir
Voxilaprevir (GS-9857)
Velpatasvir (GS-5816)
Sofosbuvir
Genotype
1a
1b
2
3
1a
1b
2
3
Treatment history
PI (NS3)-experienced
SOF-experienced
PI (NS3)-experienced
SOF-experienced
Cirrhosis
Yes
No
Yes
No
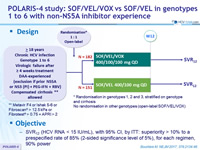
Design
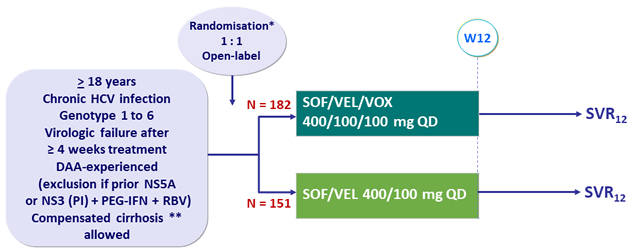
* Randomisation in genotypes 1, 2 and 3, stratified on genotype and cirrhosis
No randomisation in other genotypes (open-label SOF/VEL/VOX)
** Metavir F4 or Ishak 5-6 or Fibroscan® > 12.5 kPa or Fibrotest® > 0.75 + APRI > 2
Objective
- SVR12 (HCV RNA < 15 IU/ml) with 95% CI, by ITT: superiority > 10% to a prespecified rate of 85% (2-sided significance level of 5%), for each regimen, 90% power
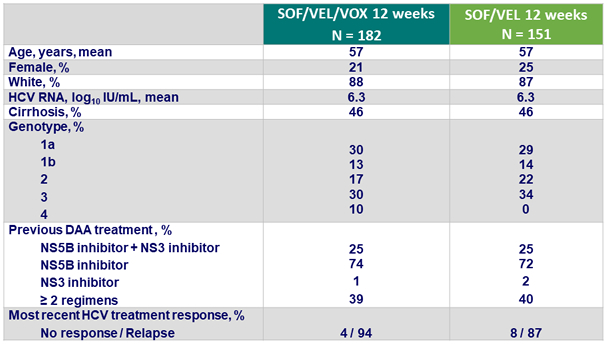
Baseline characteristics and patient disposition
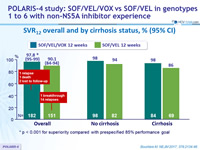
SVR12 overall and by cirrhosis status, % (95% CI)
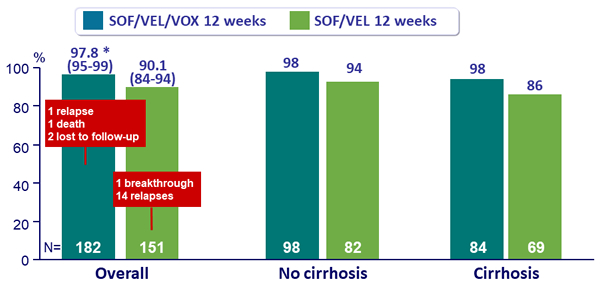
* p < 0.001 for superiority compared with prespecified 85% performance goal
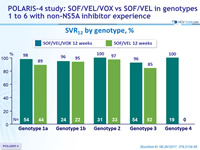
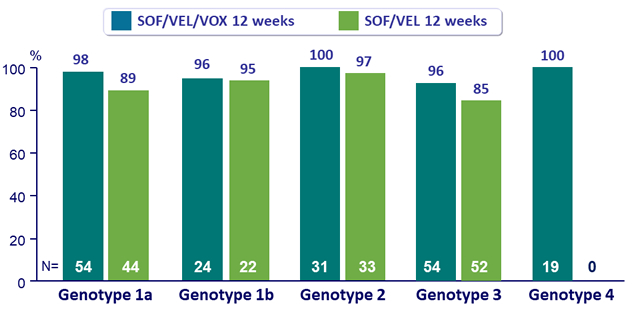
SVR12 by genotype, %
SVR12 according to baseline RASs (15% cutoff)
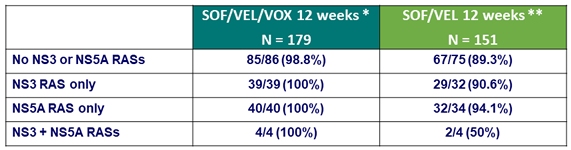
* All 22 patients with baseline NS5B RAS achieved SVR12 ; No treatment-emergent RASs in the patient who relapsed
** All 8 patients with baseline NS5B RAS achieved SVR12 ; 11/14 patients with virologic relapse developed Y93H or Y93C
Adverse events
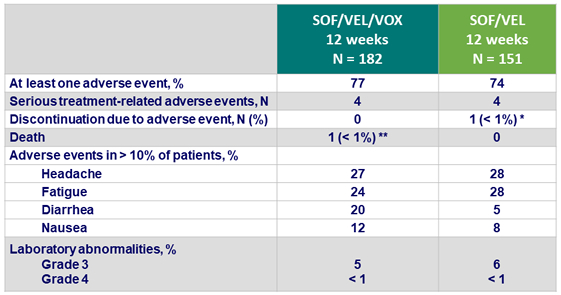
*1 patient discontinued due to worsening headaches on study D49
** 1 patient died of an opiate overdose on post-treatment D2
Summary
- In a wide variety of DAA-experienced patients, excluding those
pre-treated with NS5A inhibitor, with genotypes 1, 2, 3 or 4,
- SVR12 was 98% for 12 weeks of SOF/VEL/VOX, meeting superiority criteria to prespecified 85% rate
- SVR12 was 90% for 12 weeks of SOF/VEL
- Lower SVR12 rate in cirrhotic patients (86% vs 96%)
- Baseline RASs did not impact outcome for SOF/VEL/VOX : SVR12 rates of 100%
- No treatment-emergent RASs in the patient who relapsed with SOF/VEL/VOX
- 79% ( 11/14) patients with virologic failure to SOF/VEL had emergence of Y93H or Y93C
- SOF/VEL/VOX and SOF/VEL were well tolerated
- SOF/VEL/VOX for 12 weeks provides a simple, safe, and effective single tablet, once daily treatment for NS5B-experienced patients