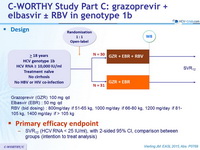
C-WORTHY Study part C: grazoprevir + elbasvir ± RBV in genotype 1b
Efficacy of an Eight-Week Regimen of Grazoprevir plus Elbasvir with and without Ribavirin in Treatment-Naive, Noncirrhotic HCV Genotype 1B Infection
Vierling JM. EASL 2015, Abs . P0769
Anti-HCV
Grazoprevir
Elbasvir
Ribavirin
Grazoprevir
Elbasvir
Ribavirin
Genotype
1b
1b
Treatment history
Naive
Naive
Cirrhosis
No
No
Design
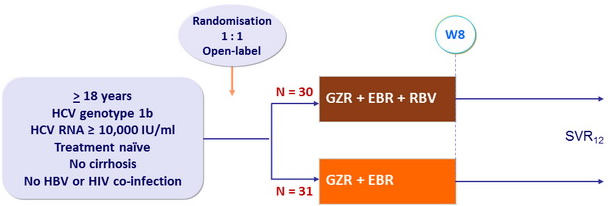
Grazoprevir (GZR) 100 mg qd
Elbasvir (EBR) : 50 mg qd
RBV (bid dosing) : 800mg/day if 51-65 kg, 1000 mg/day if 66-80 kg, 1200 mg/day if 81-105 kg, 1400 mg/day if > 105 kg
Primary efficacy endpoint
- SVR12 (HCV RNA < 25 IU/ml), with 2-sided 95% CI, comparison between groups (intention to treat analysis) Treatment groups
Baseline characteristics and patient disposition
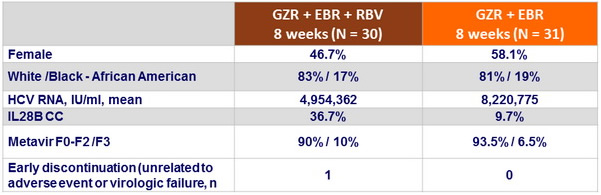
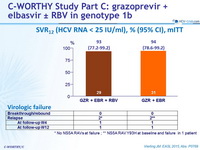
SVR12 (HCV RNA < 25 IU/ml), % (95% CI), mITT
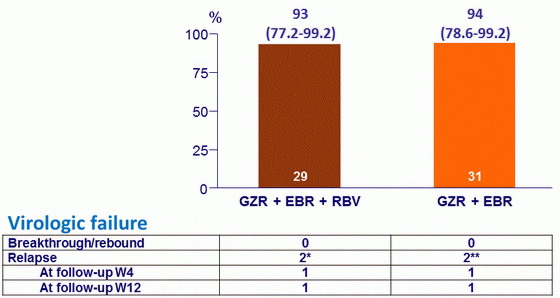
* No NS5A RAVs at failure ; ** NS5A RAV Y93H at baseline and failure in 1 patient
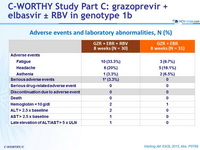
Adverse events and laboratory abnormalities, N (%)
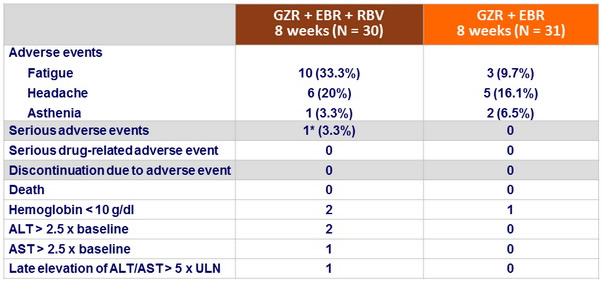
Summary
- GZR + EBR ± RBV for 8 weeks in treatment-naïve , non-cirrhotic patients with HCV genotype 1b infection was
- highly efficacious
- safe and well-tolerated
- rarely associated with resistance-associated variants at the time of failure