EXPEDITION-2 Study: glecaprevir/pibrentasvir in patients with HIV co-infection
Rockstroh J. Clin Infect Dis 2018;67:1000-17
Anti-HCV
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Genotype
1
2
3
4
1
2
3
4
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
Special population
HIV co-infection
HIV co-infection
Design
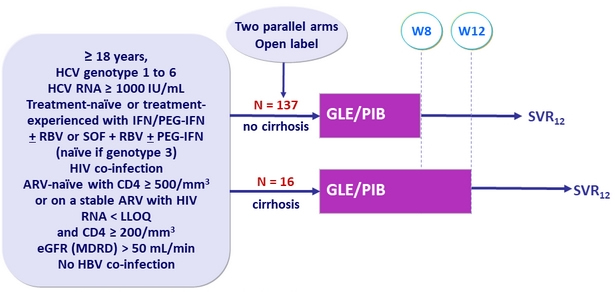
- GLE/PIB: 100/40 mg 3 tablets QD
Objective
- SVR12 (HCV RNA< 15 IU /ml)
Baseline characteristics and SVR12
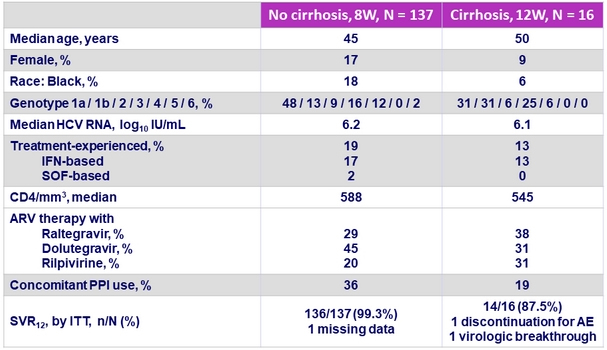
One patient with Genotype 3a infection and cirrhosis had on-treatment virologic failure
- At treatment W8
- NS3 RASs
- no polymorphisms at baseline
- Y56H at failure
- NS5A RASs
- A30V at baseline
- S24F + M28K at failure
HIV RNA suppression
- No virologic breakthrough
Adverse events and laboratory abnormalities, %
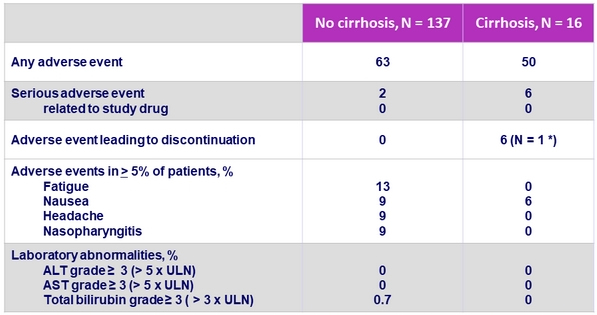
* Cerebrovascular accident and hemorrhage, unrelated to treatment ; treatment discontinuation on D23
Summary
- GLE/PIB (300 mg/120 mg QD) achieved a SVR of 98% in HCV infected patients with HIV infection,
- After 8 weeks of therapy if no cirrhosis (SVR12 of 99.3%)
- After 12 weeks of therapy if cirrhosis
- SVR12 was not impacted by baseline HCV viral load or other baseline factors
- GLE/PIB was well tolerated with a favorable safety profile in patients with or without cirrhosis
- No drug-related serious adverse event
- No grade = 3 laboratory abnormalities in ALT or AST





