C-CORAL Study: elbasvir/grazoprevir for genotype 1, 4, 6
Wei L. J Gastroenterol Hepatol. 2019; 34:12-21
Anti-HCV
Grazoprevir
Elbasvir
Grazoprevir
Elbasvir
Genotype
1
1b
6
1
1b
6
Treatment history
Naive
Naive
Cirrhosis
Yes
No
Yes
No
Design
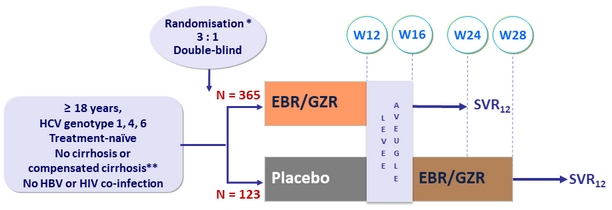
* Randomisation was stratified by cirrhosis status (yes vs no) and study site (country)
** Fibroscan® > 12.5 kPa, or liver biopsy (F4), or Fibrotest® > 0.75 or APRI > 2
- EBR/GZR: 50/100 mg 1 tablet QD
Objective
- SVR12 (HCV RNA < 15 IU/mL), by ITT
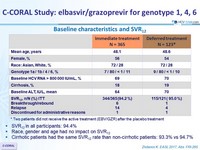
Baseline characteristics and SVR12
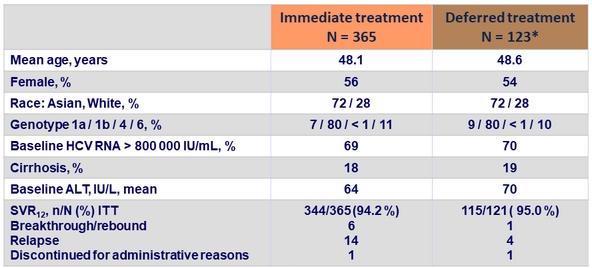
* Two patients did not receive the active treatment (EBV/GZR) after the placebo treatment
- SVR12 in all participants: 94.4%
- Race, gender and age had no impact on SVR12
- Cirrhotic patients had the same SVR12 rate than non-cirrhotic patients: 93.3% vs 94.7%
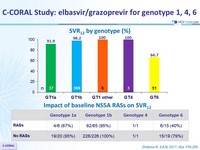
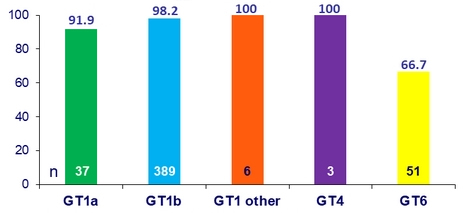
SVR12 by genotype (%)
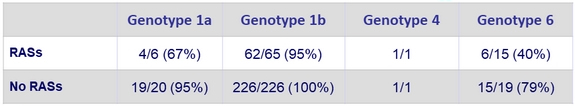
Impact of baseline NS5A RASs on SVR12
Adverse events and laboratory abnormalities, N (%)
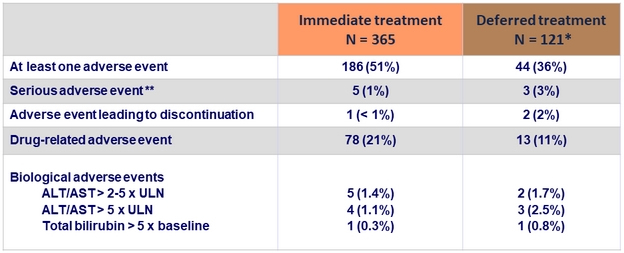
* Data given for the EBR/GZR period
** Suicide, Evan's syndrome, contusion, enteritis, gastric lymphoma, atrial fibrillation, ankle fracture, uterine hemorrhage. Only atrial fibrillation was considered related to EBR/GZR
Summary
- Treatment with a 12-week regimen of EBR/GZR achieved a global rate of SVR12 of 94% in a heterogeneous population with GT1, 4 and 6
- Low rates of SVR12 were observed in GT6 patients (66.7%)
- Cirrhosis, gender and race had no impact on SVR12
- RASs decreased SSVR12 rate in genotypes 1a and 6
- Treatment was safe and well tolerated