Sofosbuvir/velpatasvir for 12 weeks in liver transplant recipients with genotype 1-4
Agarwal K. J Hepatol. 2018; 69:603-7
Anti-HCV
Velpatasvir (GS-5816)
Sofosbuvir
Velpatasvir (GS-5816)
Sofosbuvir
Genotype
1
3
1
3
Treatment history
IFN-Experienced
IFN-Experienced
Cirrhosis
No
No
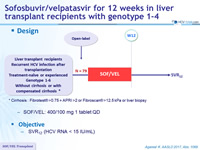
Design
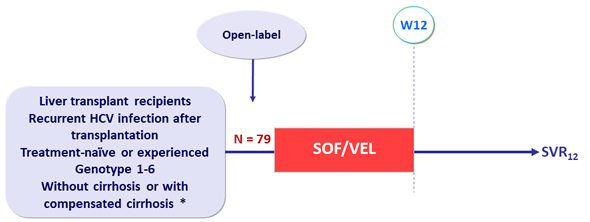
* Cirrhosis : Fibrotest ® >0.75 + APRI >2 or Fibroscan ® >12.5 kPa or liver biopsy
- SOF/VEL: 400/100 mg 1 tablet QD
Objective
- SVR12 (HCV RNA < 15 IU/mL)
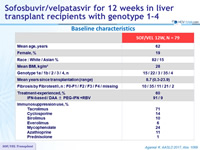
Baseline characteristics
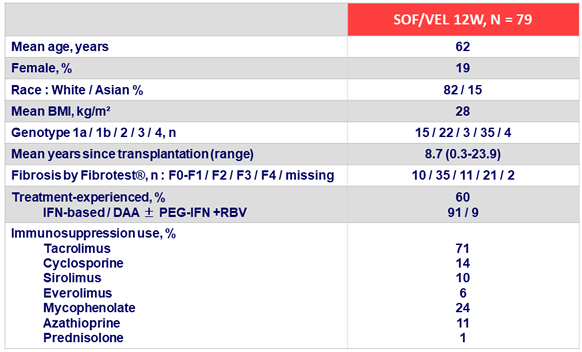
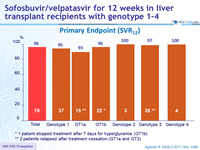
Primary Endpoint (SRV12)
* 1 patient stopped treatment after 7 days for hyperglycemia (GT1b)
** 2 patients relapsed after treatment cessation (GT1a and GT3)
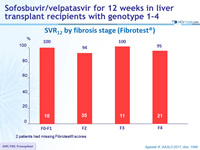
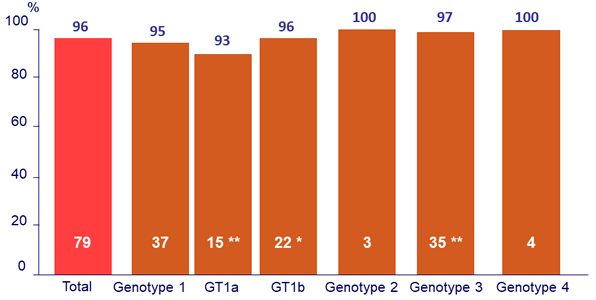
SVR12 by fibrosis stage (Fibrotest ®)
2 patients had missing Fibrotest ® scores
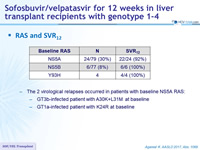
RAS and SVR12
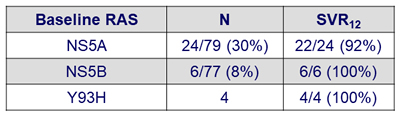
- The 2 virological relapses occurred in patients with baseline NS5A RAS:
- GT3b-infected patient with A30K+L31M at baseline
- GT1a-infected patient with K24R at baseline
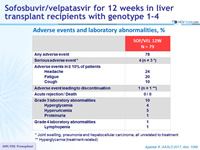
Adverse events and laboratory abnormalities, %
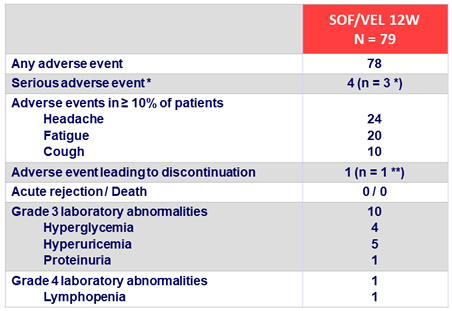
* Joint swelling, pneumonia and hepatocellular carcinoma, all unrelated to treatment
** Hyperglycemia (treatment-related)
Summary
- 12 weeks of SOF/VEL achieved high cure rate (SVR12 of 96%) in liver transplantation recipients with relapse of HCV infection with genotypes 1-4
- Good safety profile
- No rejection episode