COMMAND GT2/3 Study: daclatasvir + PEG-IFN + RBV for genotype 2 or 3
Daclatasvir Plus Peginterferon and Ribavirin Is Noninferior to Peginterferon and Ribavirin Alone, and Reduces the Duration of Treatment for HCV Genotype 2 or 3 Infection
Dore GJ. Gastroenterology 2015;148:355-66
Anti-HCV
Daclatasvir
PEG-IFNα 2a
Ribavirin
Daclatasvir
PEG-IFNα 2a
Ribavirin
Genotype
2
3
2
3
Treatment history
Naive
Naive
Cirrhosis
Yes
Yes
Design
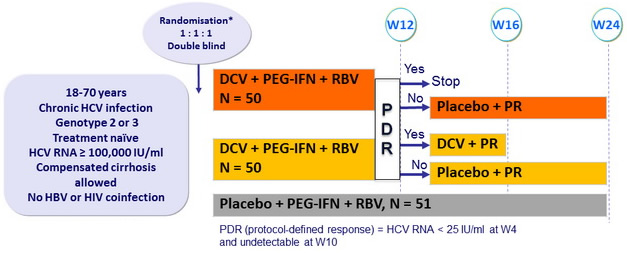
* Randomisation stratified on genotype (2 or 3)
- DCV : 60 mg qd or matching placebo (2 pills) ; PEG-IFNα-2a : 180 mg SC once weekly
- RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or ≥ 75 kg)
Objective
- SVR24 (HCV RNA undetectable) : non-inferiority of DCV regimens (lower limit of the 80% CI : - 20%), 85% power
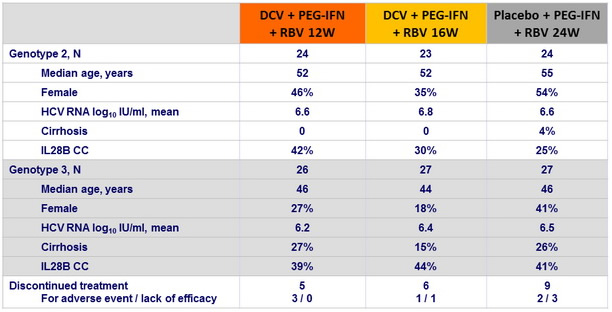
Baseline characteristics and patient disposition
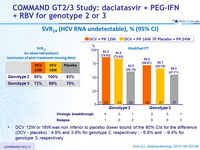
SVR24 (HCV RNA undetectable), % (95% CI)
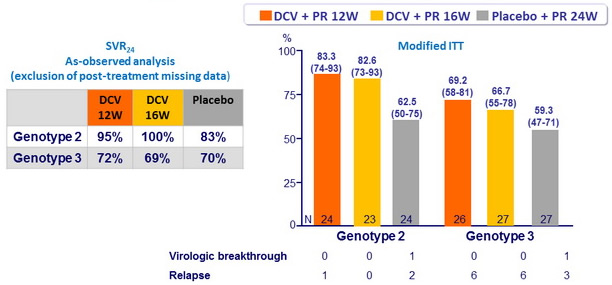
- DCV 12W or 16W was non inferior to placebo (lower bound of the 80% CIs for the difference (DCV - placebo) : 4.9% and 3.9% for genotype 2, respectively ; - 6.8% and - 9.4% for genotype 3, respectively
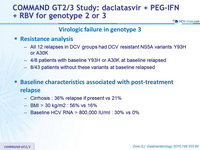
Virologic failure in genotype 3
- Resistance analysis
- All 12 relapses in DCV groups had DCV resistant NS5A variants Y93H or A30K
- 4/8 patients with baseline Y93H or A30K at baseline relapsed
- 8/43 patients without these variants at baseline relapsed
- Baseline characteristics associated with post-treatment relapse
- Cirrhosis : 36% relapse if present vs 21%
- BMI > 30 kg/m2 : 56% vs 16%
- Baseline HCV RNA > 800,000 IU/ml : 30% vs 0%
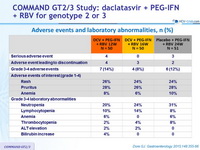
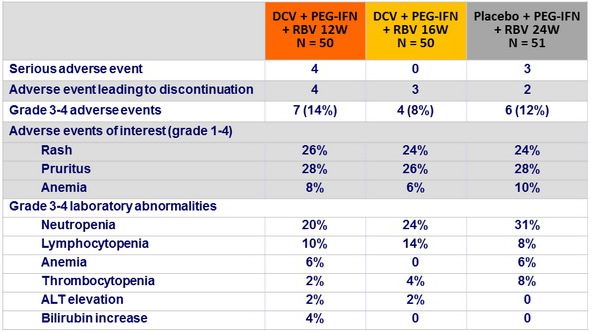
Adverse events and laboratory abnormalities, n (%)
Summary
- 12 or 16 weeks of treatment with DCV, in combination with
PEG-IFN + RBV, is a well tolerated and effective therapy for patients with HCV genotype 2 or 3 infections
- For the primary end point of SVR24 , the differences between both DCV arms and placebo met statistical criteria for noninferiority in patients with genotype 2 and genotype 3 infection
- DCV containing regimens could reduce the duration of therapy for these patients, when given with PEG-IFN + RBV
- These results suggest that combinations of DCV with other potent oral antiviral agents may offer alternatives to the current standard of care for genotypes 2 and 3 infection