SOF/VEL ± RBV in genotype 3 with compensated cirrhosis
Esteban R. Gastroenterology. 2018; 155:1120-7
Anti-HCV
Velpatasvir (GS-5816)
Sofosbuvir
Ribavirin
Velpatasvir (GS-5816)
Sofosbuvir
Ribavirin
Genotype
3
3
Treatment history
Naive
IFN-Experienced
PI (NS3)-experienced
NS5A experienced
SOF-experienced
Naive
IFN-Experienced
PI (NS3)-experienced
NS5A experienced
SOF-experienced
Cirrhosis
Yes
Yes
Special population
HIV co-infection
HIV co-infection
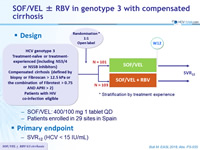
Design
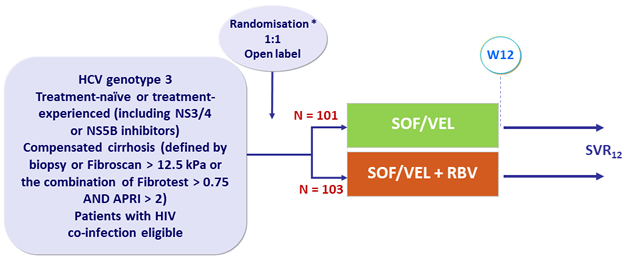
* Stratification by treatment experience
- SOF/VEL: 400/100 mg 1 tablet QD
- Patients enrolled in 29 sites in Spain
Primary endpoint
- SVR12 (HCV < 15 IU/mL)
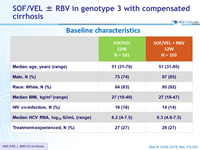
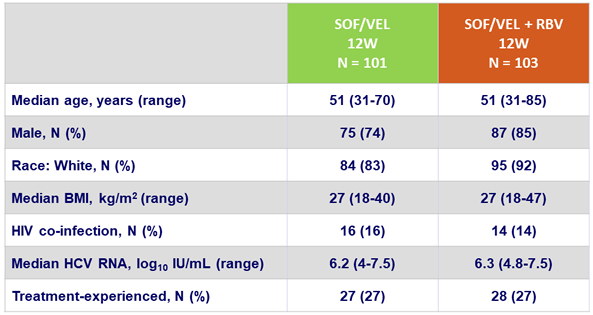
Baseline characteristics
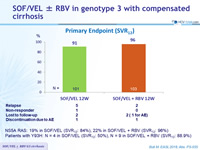
Primary Endpoint (SVR12)
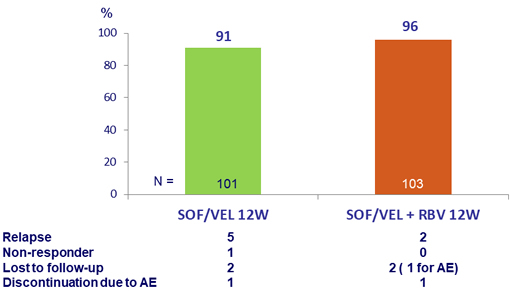
NS5A RAS: 19% in SOF/VEL (SVR12 : 84%), 22% in SOF/VEL + RBV (SVR12 : 96%)
Patients with Y93H: N = 4 in SOF/VEL (SVR12 : 50%), N = 9 in SOF/VEL + RBV (SVR12 : 88.9%)
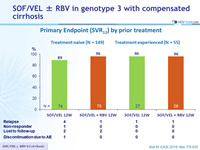
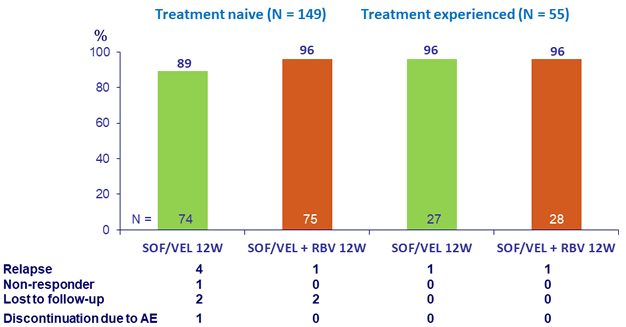
Primary Endpoint (SVR12) by prior treatment
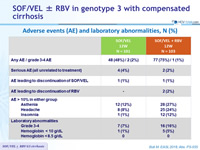
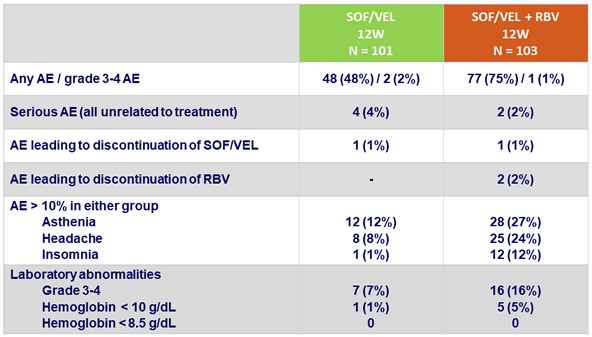
Adverse events (AE) and laboratory abnormalities, N (%)
Conclusion
- High rates of SVR12 were observed with 12 weeks of SOF/VEL ± RBV in patients with GT3 and compensated cirrhosis
- Adding RBV reduced the relapse rate from 5% to 2%
- Treatment was well-tolerated