CURRY Study: SOF + RBV for HCV with liver cancer before transplantation
Sofosbuvir and Ribavirin Prevent Recurrence of HCV Infection After Liver Transplantation: An Open-Label Study
Curry MP. Gastroenterology 2015;148:100-107
Anti-HCV
Sofosbuvir
Ribavirin
Sofosbuvir
Ribavirin
Genotype
1
1a
1b
1
1a
1b
Special population
Liver transplantation
Liver transplantation
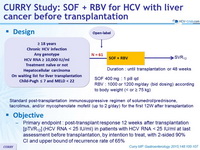
Design
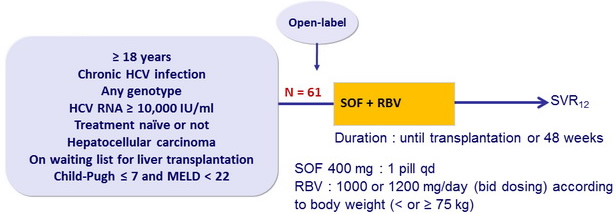
Standard post-transplantation immunosuppressive regimen of solumedrol / prednisone, tacrolimus, and/or mycophenolate mofetil (up to 2 g/day) for the first 12W after transplantation
Objective
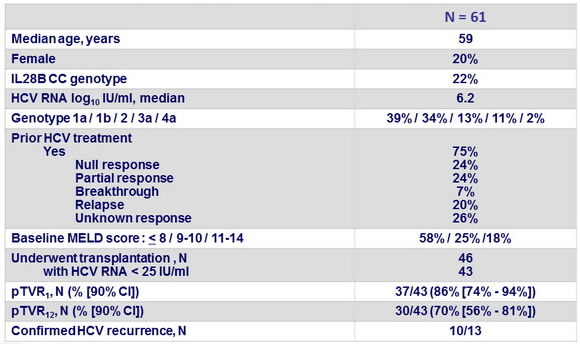
- Primary endpoint : post-transplant response 12 weeks after transplantation [pTVR 12] (HCV RNA < 25 IU/ml) in patients with HCV RNA < 25 IU/ml at last assessment before transplantation, by intention to treat, with 2 -sided 90% CI and upper bound of recurrence rate of 65% Baseline characteristics and patient disposition
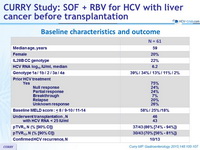
Baseline characteristics and outcome
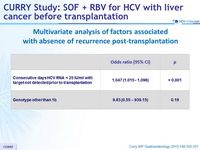
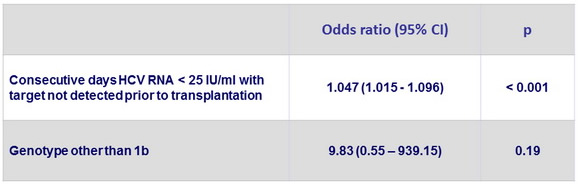
Multivariate analysis of factors associated with absence of recurrence post-transplantation
HCV Recurrence and resistance analysis
- 10 confirmed recurrence after liver transplantation
- Genotype 1a, N = 2; 1b, N = 7; 3a, N = 1
- ILB28 non-CC, N = 10
- Recurrence at W1 (N = 3), W2 (N = 2), W4 (N = 4), or W12 (N = 1) post-transplantation
- Duration of SOF+ RBV pre-transplantation < 12 weeks , N = 4
- NS5B sequencing
- At baseline :
- 4 patients with L159F variant : 4/4 relapsed
- 1 patient with N142T : achieved SVR12
- . 29 patients with failure before transplantation or recurrence after transplantation
- no S282T mutant
- 12 patients with other variants as minor subpopulations (< 10%) in 11/12 : N142T (N = 2), L159F (N = 5), S282G (N = 1), L230F
(N = 3); L159F + S282R + L230F + V321A (N = 1)
- At baseline :
Adverse events
- Median duration of exposure to study regimen : 21 weeks
- Serious adverse events, N = 11
- = Grade 3 adverse event, N = 11
- Discontinuation due to adverse event, N = 2 (pneumonia, sepsis/acute renal failure)
- Most common adverse events :
- Fatigue (38%)
- Headache (23%)
- Anemia (21%)
- Nausea (16%)
- Rash (15%)
- Dyspnea (11%)
- Cough (11%)
- Insomnia (11%)
- Constipation (10%)
- Pruritus (10%)
- Most common grade 3-4 laboratory abnormalities : grade 3 decrease in hemoglobin level, grade 3 hyperglycemia, grade 3-4 bilirubin elevation, lymphopenia < 500/mm3
- 12 patients with RBV dose reduction, but no transfusion, no epoetin needed
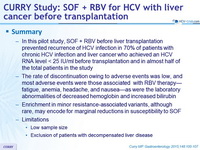
Summary
- In this pilot study, SOF + RBV before liver transplantation prevented recurrence of HCV infection in 70% of patients with chronic HCV infection and liver cancer who achieved an HCV RNA level < 25 IU/ml before transplantation and in almost half of the total patients in the study
- The rate of discontinuation owing to adverse events was low, and most adverse events were those associated with RBV therapy-fatigue, anemia, headache, and nausea-as were the laboratory abnormalities of decreased hemoglobin and increased bilirubin
- Enrichment in minor resistance-associated variants, although rare, may encode for marginal reductions in susceptibility to SOF
- Limitations
- Low sample size
- Exclusion of patients with decompensated liver disease