AI444040 Study: DCV + SOF ± RBV for genotypes 1, 2 and 3
Daclatasvir plus Sofosbuvir for Previously Treated or Untreated Chronic HCV Infection
Sulkowski MS. NEJM 2014;370:211-21
Anti-HCV
Daclatasvir
Sofosbuvir
Daclatasvir
Sofosbuvir
Genotype
1
2
3
1
2
3
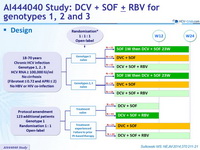
Treatment history
Naive
PI (NS3)-experienced
Naive
PI (NS3)-experienced
Cirrhosis
No
No
Design
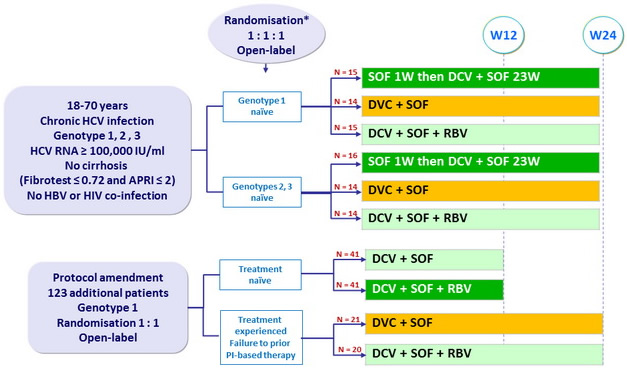
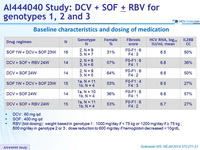
Baseline characteristics and dosing of medication
- DCV : 60 mg qd
- SOF : 400 mg qd
- RBV (bid dosing) : weight based in genotype 1 : 1000 mg/day if < 75 kg or 1200 mg/day if = 75 kg ; 800 mg/day in genotype 2 or 3 ; dose reduction to 600 mg/day if hemoglobin decreased < 10g/ dL
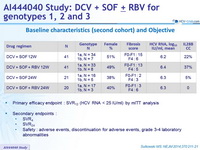
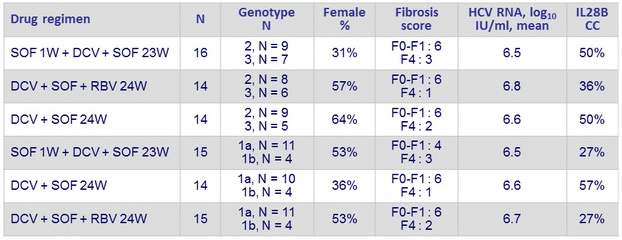
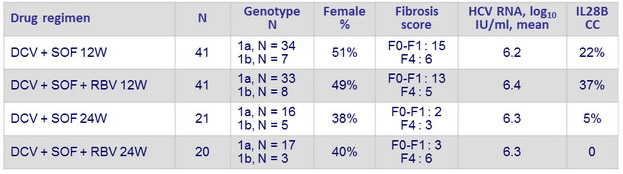
Baseline characteristics (second cohort) and Objective
- Primary efficacy endpoint : SVR12 (HCV RNA < 25 IU/ml) by mITT analysis
- Secondary endpoints :
- SVR4
- SVR24
- Safety : adverse events, discontinuation for adverse events, grade 3-4 laboratory abnormalities
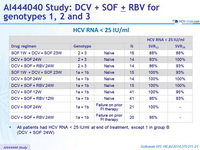
HCV RNA < 25 IU/ml
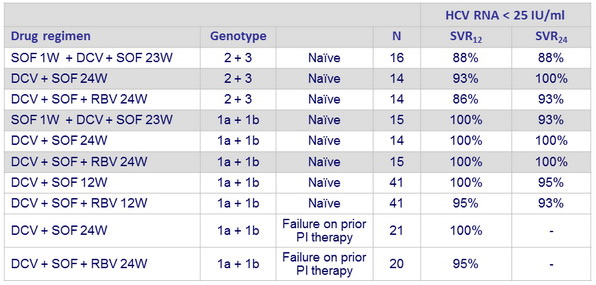
- All patients had HCV RNA < 25 IU/ml at end of treatment , except 1 in group B (DCV + SOF 24W)
Virologic relapse post-treatment : 1 patient with genotype 3 who did not received RBV
- NS5A A30K polymorphism (DCV resistance) at baseline and failure
Resistance testing (sequencing)
- NS5A polymorphisms associated with loss of susceptibility to DCV in vitro detected at baseline in 32 patients : 8% in genotype 1, 61% in genotype 2, 28% in genotype 3
- Most frequent mutations : Q30H (genotype 1a), L31M
(genotype 1b and 2), Y93H (genotype 3) - Except the patient with relapse, all other patients with preexisting DCV resistant variants had a SVR
- No mutation (S282T) to SOF at baseline or in the patient with breakthrough
Adverse events , n (%)
- Most common : fatigue, headache, nausea (= 25% in any group)
- Grade 3-4 adverse events : 7 (3.3%)
- Discontinuation of treatment for adverse events : 2
(both achieved SVR)
- DCV-SOF 24W : 1 cerebrovascular accident
- DCV-SOF + RBV 24W : 1 fibromyalgia exacerbation
- Serious adverse events : 10 (4.7%)
- Most common grade 3-4 laboratory abnormalities : low phosphorus and elevation of glucose levels
- Hemoglobin level more reduced in groups with RBV
- Reduction of RBV dose in 5 patients because of anemia
Summary
- DCV + SOF was assessed in untreated patients and patients in whom previous treatment with telaprevir or boceprevir had failed
- Most patients had a SVR,
- including 98% of patients with genotype 1 infection, regardless of viral subtype or failure of prior treatment with PI,
- and 91% of naïve patients infected with genotype 2 or 3
- The most common adverse event was fatigue, which was reported in approximately one third of patients
- Virologic breakthrough and relapse were rare and were not observed in any of the patients infected with HCV genotype 1 or 2, despite preexisting DCV-resistant variants in 14%
- In genotype 3, 1 relapse in a patient with baseline DCV-resistant variant
- No additional benefit of RBV addition but greater decrease in hemoglobin