ELECTRON Study: SOF-based therapy for genotypes 1, 2 and 3
Nucleotide Polymerase Inhibitor Sofosbuvir plus Ribavirin for Hepatitis C
Gane EJ. NEJM 2013;368:34-44
Anti-HCV
Sofosbuvir
Sofosbuvir
Genotype
1
1a
2
3
1
1a
2
3
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
No
No
Design

Objective
- SVR24 with two-sided 95% CI, descriptive analysis
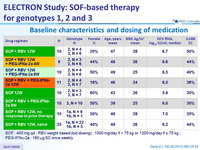
Baseline characteristics and dosing of medication
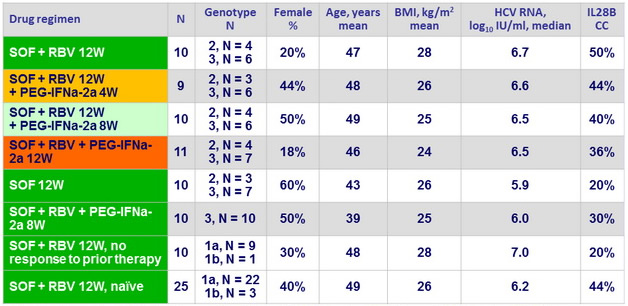
SOF : 400 mg qd ; RBV weight based ( bid dosing ) : 1000 mg/ day if < 75 kg or 1200 mg/ day if = 75 kg ;
PEG-IFNα-2a : 180 m g SC once weekly
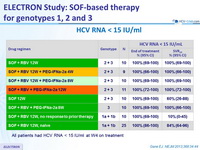
HCV RNA < 15 IU/ml
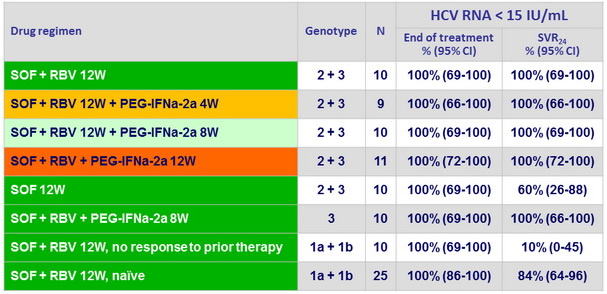
All patients had HCV RNA < 15 IU/ml at W4 on treatment
Resistance testing (sequencing)
- 4 relapse (2 genotype 2 and 2 genotype 3) in the SOF 12W group
- 1 patient with S282T mutation (genotype 2b)
- No NS5B substitutions in the other 3 patients
- 15 relapse in genotype 1 patients
- No NS5B substitutions
Adverse events
- Most common : headache, fatigue, insomnia, nausea, rash, anemia
- IFN-free groups : less severe reductions in hemoglobin, no neutropenia, no thrombopenia
- SOF monotherapy : mean decrease of 0.54g/dl of hemoglobin
Summary
- By W4 of treatment, all 95 patients in the study had an undetectable level of HCV RNA
- All 50 previously untreated patients with HCV genotype 2 or 3 infection who received 8 or 12 weeks of treatment with SOF + RBV, with or without PEG-IFN- alfa 2a, had a sustained virologic response at 24 weeks after treatment
- SOF alone 12W was associated with 40% of relapse, suggesting a role for RBV in genotype 2 or 3 to maintain antiviral response
- In genotype 1, SVR was much higher in patients naïve to treatment
- There was no discontinuation of SOF or RBV in any group
- Sofosbuvir plus ribavirin for 12 weeks may be effective in previously untreated patients with HCV genotype 1, 2, or 3 infection