ION-3 Study: LDV/SOF ± RBV for naïve genotype 1
Ledipasvir and Sofosbuvir for 8 or 12 Weeks for Chronic HCV without Cirrhosis
Kowdley KV. NEJM 2014;370:1879-88
Anti-HCV
Ledipasvir
Sofosbuvir
Ribavirin
Ledipasvir
Sofosbuvir
Ribavirin
Genotype
1
1a
1b
1
1a
1b
Treatment history
Naive
Naive
Cirrhosis
No
No
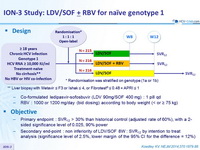
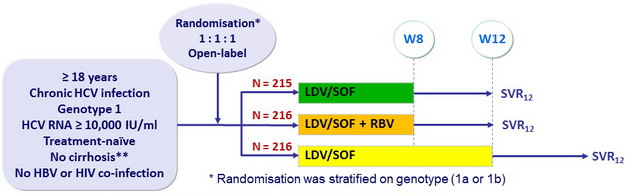
Design
** Liver biopsy with Metavir = F3 or Ishak = 4, or Fibrotest ® = 0.48 + APRI = 1
- Co-formulated ledipasvir-sofosbuvir (LDV 90mg/SOF 400 mg) : 1 pill qd
- RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or ≥ 75 kg)
Objective
- Primary endpoint : SVR12 > 30% than historical control (adjusted rate of 60%), with a 2-sided significance level of 0.025, 90% power
- Secondary end-point : non inferiority of LDV/SOF 8W : SVR12 by intention to treat analysis (significance level of 2.5%, lower margin of the 95% CI for the difference = 12%)
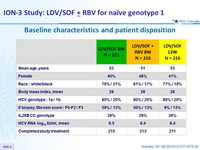
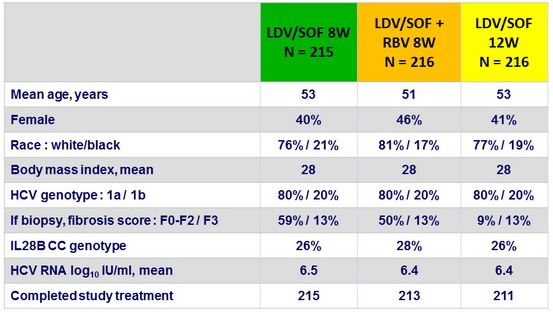
Baseline characteristics and patient disposition
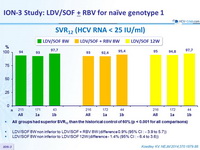
SVR12 (HCV RNA < 25 IU/ mL)
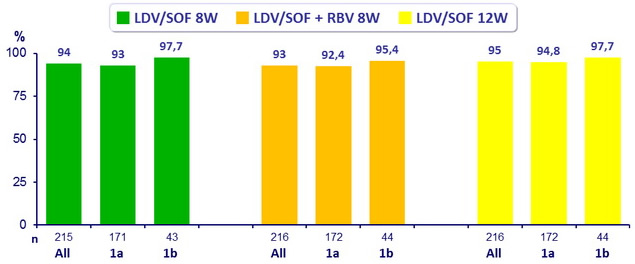
- All groups had superior SVR12 than the historical control of 60% (p < 0.001 for all comparisons)
- LDV/SOF 8W non inferior to LDV/SOF + RBV 8W (difference 0.9% (95% CI : - 3.9 to 5.7 ))
- LDV/SOF 8W non inferior to LDV/SOF 12W (difference - 1.4% (95% CI : - 6.4 to 3.6 ))
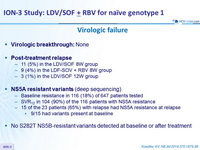
Virologic failure
- Virologic breaktrough : None
- Post-treatment relapse
- 11 (5%) in the LDV/SOF 8W group
- 9 (4%) in the LDF-SOV + RBV 8W group
- 3 (1%) in the LDV/SOF 12W group
- NS5A resistant variants
(deep sequencing)
- Baseline resistance in 116 (18%) of 647 patients tested
- SVR12 in 104 (90%) of the 116 patients with NS5A resistance
- 15 of the 23 patients (65%) with relapse had NS5A resistance at relapse
- 9/15 had variants present at baseline
- No S282T NS5B-resistant variants detected at baseline or after treatment
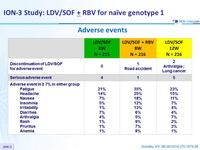
Adverse events
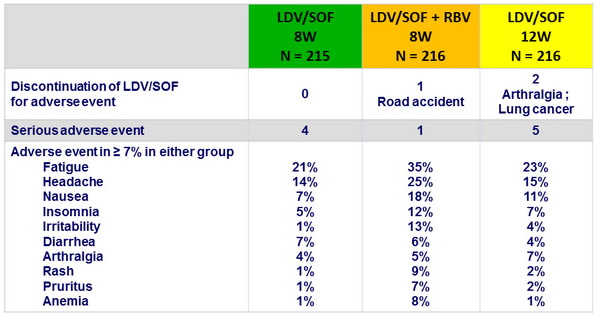
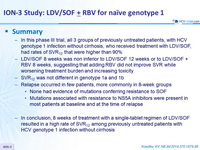
Summary
- In this phase III trial, all 3 groups of previously untreated patients, with HCV genotype 1 infection without cirrhosis, who received treatment with LDV/SOF, had rates of SVR12 that were higher than 90%
- LDV/SOF 8 weeks was non inferior to LDV/SOF 12 weeks or to LDV/SOF + RBV 8 weeks, suggesting that adding RBV did not improve SVR while worsening treatment burden and increasing toxicity
- SVR12 was not different in genotype 1a and 1b
- Relapse occurred in few patients, more commonly in 8-week groups
- None had evidence of mutations conferring resistance to SOF
- Mutations associated with resistance to NS5A inhibitors were present in most patients at baseline and at the time of relapse
- In conclusion, 8 weeks of treatment with a single-tablet regimen of LDV/SOF resulted in a high rate of SVR12 among previously untreated patients with HCV genotype 1 infection without cirrhosis