LONESTAR-2 Study: SOF + PEG-IFN + RBV for genotypes 2 and 3 in prior failure
Sofosbuvir With Peginterferon-Ribavirin for 12 Weeks in Previously Treated Patients With Hepatitis C Genotype 2 or 3 and Cirrhosis
Lawitz E. Hepatology 2015;61:769-75
Anti-HCV
Sofosbuvir
PEG-IFNα 2a
Ribavirin
Sofosbuvir
PEG-IFNα 2a
Ribavirin
Genotype
2
3
2
3
Treatment history
IFN-Experienced
IFN-Experienced
Design
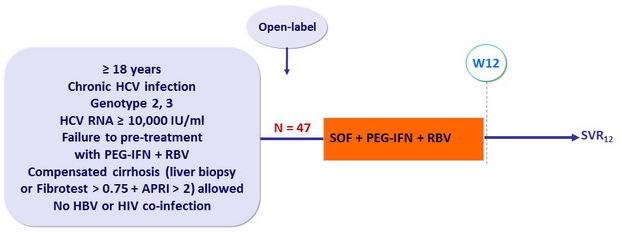
- SOF 400 mg : 1 pill qd ; PEG-IFNα-2a : 180 m g SC once weekly
- RBV : 1000 or 1200 mg/ day ( bid dosing ) according to body weight (< or = 75 kg)
Objective
- Primary endpoint : SVR12 (HCV RNA < 15 IU/ml) by intention to treat, with 2-sided 95% CI, no statistical hypothesis
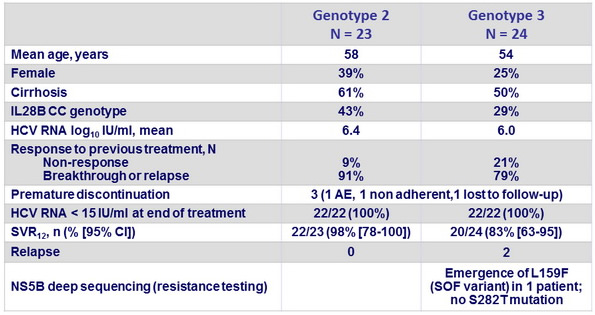
Baseline characteristics, patient disposition and outcome
Adverse events
- Serious adverse events : 5 in 4 patients
- Discontinuation of RBV in 3 patients, because of anemia
- Discontinuation of all therapy in 1 patient, because of pain
- Most common adverse events (> 20%) :
- Influenza-like illness
- Fatigue
- Anemia
- Neutropenia
- Grade 3 elevations in bilirubin in 4 patients, of whom 3 had cirrhosis
Summary
- This phase II study was the first to evaluate the efficacy, safety, and tolerability of 12-week administration of SOF + PEG-IFN + RBV in treatment-experienced patients with genotype 2 and 3 HCV infection, with and without cirrhosis
- This study showed high SVR rates in patients who have historically exhibited suboptimal response rates to HCV treatment
- SVR12 and SVR24 rates were similar
- 2 relapses occurred in patients with genotype 3
- There was no clinically significant treatment-emergent safety issues related to SOF
- Limitations
- Small size
- Study not powered