ALLY-1 Study: DCV + SOF + RBV for advanced liver disease and post-liver transplant recurrence
Daclatasvir, Sofosbuvir, and Ribavirin Combination for HCV Patients with Advanced Cirrhosis or Posttransplant Recurrence: PHASE 3
Poordad F. Hepatology 2016; 63: 1493-505
Anti-HCV
Daclatasvir
Sofosbuvir
Ribavirin
Daclatasvir
Sofosbuvir
Ribavirin
Genotype
1
1a
1b
3
1
1a
1b
3
Cirrhosis
Yes
Yes
Special population
Liver transplantation
Liver transplantation
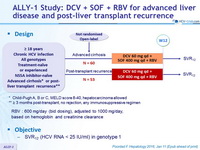
Design
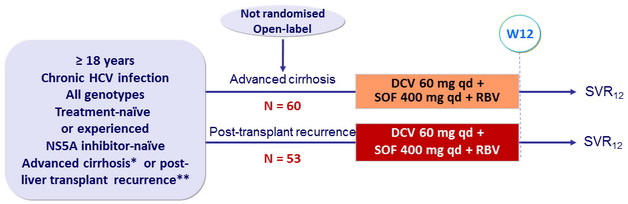
* Child-Pugh A, B or C, MELD score 8-40, hepatocarcinoma allowed
** = 3 months post-transplant, no rejection, any immunosuppressive regimen
RBV : 600 mg/day (bid dosing), adjusted to 1000 mg/day, based on hemoglobin and creatinine clearance
Objective
- SVR12 (HCV RNA < 25 IU/ml) in genotype 1
Baseline characteristics
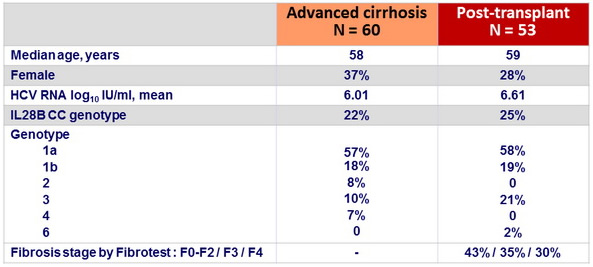
Advanced cirrhosis cohort
- Child Pugh A = 12 (MELD score 10-15 : 5/12)
- B = 32 (2/3 with ascites and/or encephalopathy , MELD score 10-20 : 25/32)
- C = 16 (all with ascites and encephalopathy ; MELD score = 16 : 13/16)
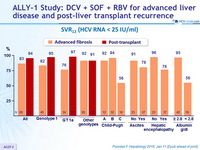
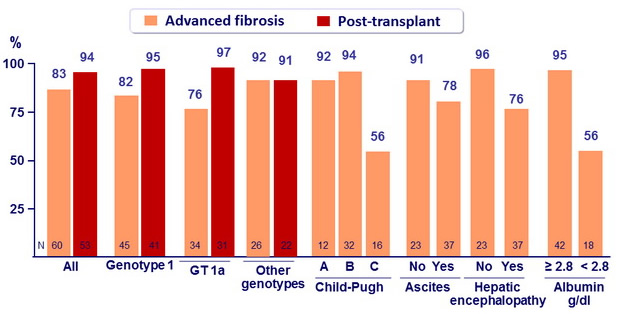
SVR12 (HCV RNA < 25 IU/ml)
Baseline resistance polymorphisms
- NS5A variants (-28, -30, -31, or -93 polymorphisms) detected in 22 of 112 patients
- 82% (18/22) achieved SVR12
- 10/14 in cirrhosis cohort ; 8/8 in post-transplant cohort
- 90% (81/90) without NS5A polymorphisms achieved SVR12
- 39/45 in cirrhosis cohort ; 42/45 in post-transplant cohort
- 82% (18/22) achieved SVR12
- No NS5B-S282 variants detected at baseline or failure
NS5A resistance-associated variants in patients with virologic failure
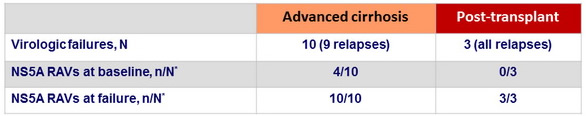
* Assessed by population-based sequencing
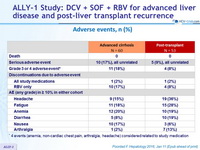
Adverse events, n (%)
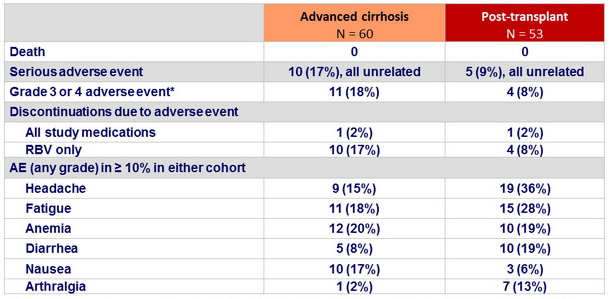
* 4 events (anemia, non-cardiac chest pain, arthralgia, headache) considered related to study medication
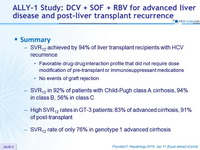
Summary
- SVR12 achieved by 94% of liver transplant recipients with HCV recurrence
- Favorable drug-drug interaction profile that did not require dose modification of pre-transplant or immunosuppressant medications
- No events of graft rejection
- SVR12 in 92% of patients with Child-Pugh class A cirrhosis, 94% in class B, 56% in class C
- High SVR12 rates in GT-3 patients: 83% of advanced cirrhosis, 91 % of post- transplant
- SVR12 rate of only 76% in genotype 1 advanced cirrhosis