NIAID ERADICATE Study: LDV/SOF for genotype 1 in HIV co-infection
Virologic Response Following Combined Ledipasvir and Sofosbuvir Administration in Patients With HCV Genotype 1 and HIV Co-infection
Osinusi A. JAMA 2015; 313:1232-9
Anti-HCV
Ledipasvir
Sofosbuvir
Ledipasvir
Sofosbuvir
Genotype
1
1a
1b
1
1a
1b
Treatment history
Naive
Naive
Cirrhosis
No
No
Special population
HIV co-infection
HIV co-infection
Design
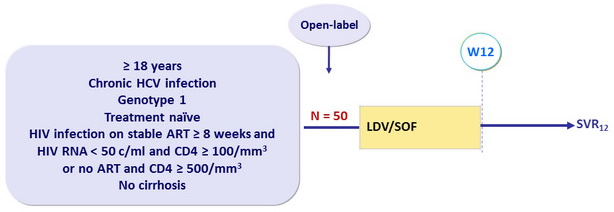
Acceptable ARV : FTC/TDF + EFV or RAL or RPV
LDV/SOF 90mg/400 mg : 1 pill qd
Objective
- Primary endpoint : SVR12 (HCV RNA < 12 IU/ml) by intention to treat, with 2-sided 95% CI
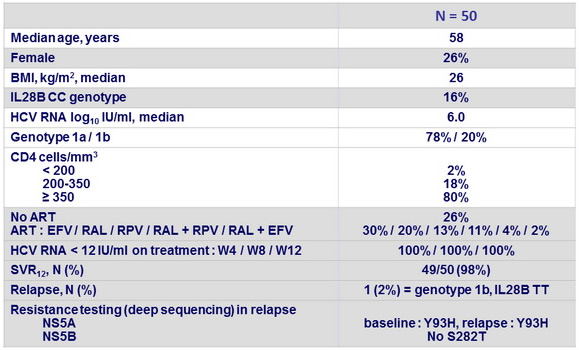
Baseline characteristics and outcome
Adverse events , n (%)
- Serious adverse events : 1 (pneumonia)
- No discontinuation due to adverse event
- Common adverse events, mostly grade 1 :
- Nasal congestion 16%)
- Myalgia (14%)
- Headache (10%)
- Fatigue (10%)
- Diarrhea (8%)
- Nausea (6 %)
- Constipation (6%)
- Urinary tract infection (6%)
- Grade 4 events : 4 (pneumonia, decrease neutrophil count, elevated AST, CK elevation)
- No significant changes in serum creatinine or eGFR
Summary
- In this open- label, uncontrolled, non randomised study, LDV/SOF single tablet regimen was associated with high rates of SVR in patients with HCV genotype 1 and HIV co-infection, similar to that observed inpatients monoinfected with HCV genotype 1
- Most adverse events were mild (grade 1-2) and clinically manageable
- Limitations
- Low sample size
- Exclusion of cirrhosis
- Restriction in antiretroviral regimen (exclusion of PI and NNRTI)
- Exclusion of patients with low CD4 cell counts