POSITRON Study: SOF + RBV for HCV genotypes 2 and 3
Sofosbuvir for Hepatitis C Genotype 2 or 3 in Patients without Treatment Options
Jacobson IM. NEJM 2013; 368:1867-77
Anti-HCV
Sofosbuvir
Ribavirin
Sofosbuvir
Ribavirin
Genotype
2
3
2
3
Treatment history
IFN-Experienced
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
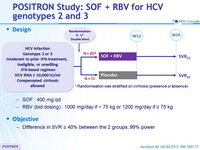
Design
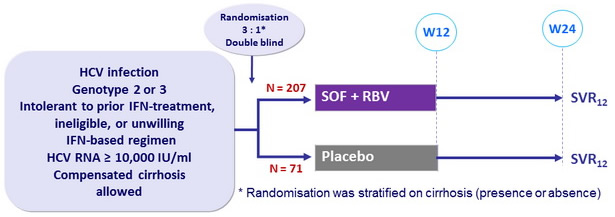
Treatment regimens
- SOF : 400 mg qd
- RBV (bid dosing) : 1000 mg/day if < 75 kg or 1200 mg/day if = 75 kg
Objective
- Difference in SVR = 40% between the 2 groups, 99% power
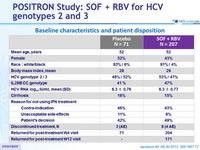
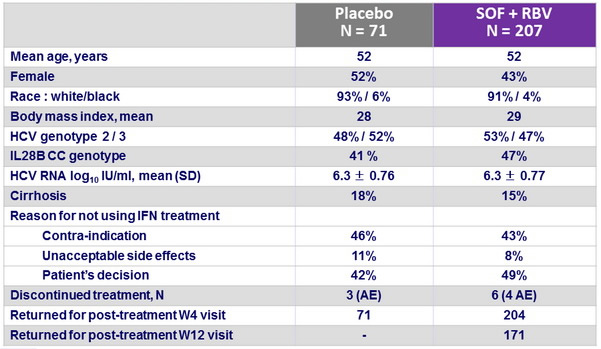
Baseline characteristics and patient disposition
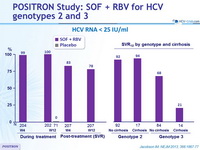
HCV RNA < 25 IU/ml
- Virologic breakthrough during treatment : none
- Relapse in patients with HCV RNA < 25 IU/ml at end of completed treatment
- 40/201 (20%) in patients who completed treatment
- 2/4 (50%) in patients who did not complete treatment
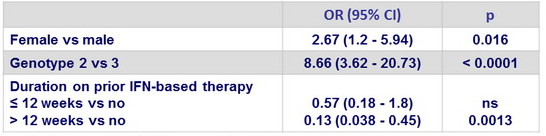
Multivariate analysis of factors associated with SVR12 in genotype 1
- Resistance testing (sequencing)
- Done in 39/42 relapses
- No SOF-associated mutation (S282T)
- 5 NS5B substitutions in > 2 subjects (no change in susceptibility to SOF)
- Done in 39/42 relapses
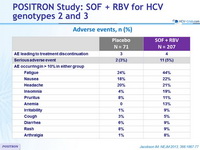
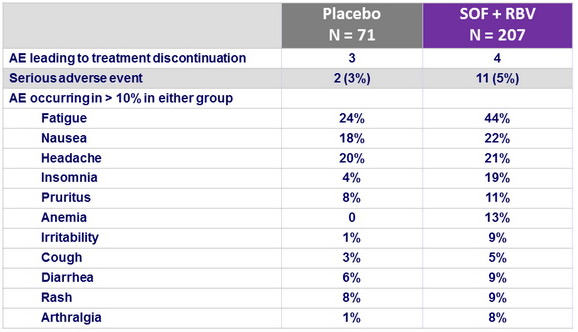
Adverse events, n (%)
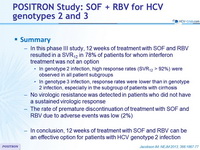
Summary
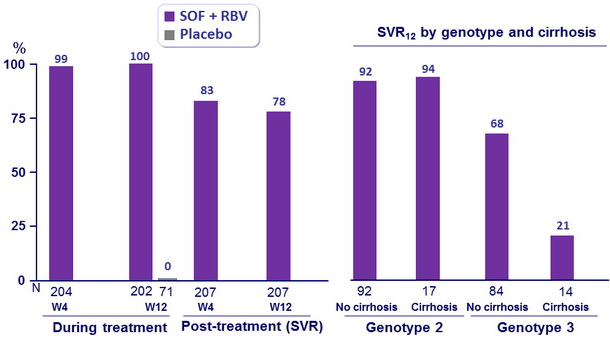
- In this phase III study, 12 weeks of treatment with SOF and RBV resulted in a SVR12 in 78% of patients for whom interferon treatment was not an option
- In genotype 2 infection, high response rates (SVR12 > 92%) were observed in all patient subgroups
- In genotype 3 infection, response rates were lower than in genotype 2 infection, especially in the subgroup of patients with cirrhosis
- No virologic resistance was detected in patients who did not have a sustained virologic response
- The rate of premature discontinuation of treatment with SOF and RBV due to adverse events was low (2%)
- In conclusion, 12 weeks of treatment with SOF and RBV can be an effective option for patients with HCV genotype 2 infection