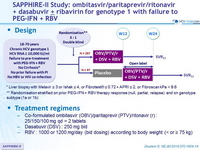
SAPPHIRE-II Study: ombitasvir/paritaprevir/ritonavir + dasabuvir ± ribavirin for genotype 1 with failure to PEG-IFN + RBV
Retreatment of HCV with ABT-450/r–Ombitasvir and Dasabuvir with Ribavirin
Zeuzem S. NEJM 2014;370:1604-14
Anti-HCV
Ombitasvir
Paritaprevir/ritonavir
Dasabuvir
Ribavirin
Ombitasvir
Paritaprevir/ritonavir
Dasabuvir
Ribavirin
Genotype
1
1a
1b
1
1a
1b
Treatment history
IFN-Experienced
IFN-Experienced
Cirrhosis
No
No
Design
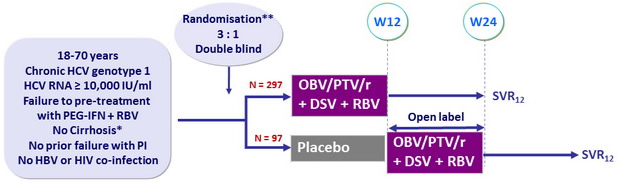
* Liver biopsy with Metavir ≤ 3 or Ishak ≤ 4, or Fibrotest® ≤ 0.72 + APRI ≤ 2, or Fibroscan kPa < 9.6
** Randomisation stratified on prior PEG-IFN + RBV therapy response (null, partial, relapse) and on genotype
subtype (1a or 1b)
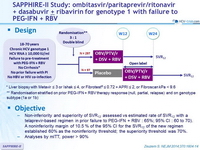
Treatment regimens
- Co-formulated ombitasvir (OBV)/paritaprevir (PTV)/ritonavir (r) : 25/150/100 mg qd = 2 tablets
- Dasabuvir (DSV) : 250 mg bid
- RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or ≥ 75 kg)
Objectives
- Non-inferiority and superiority of SVR12 assessed vs estimated rate of SVR12 with a telaprevir-based regimen in prior failure to PEG-IFN + RBV : 65%; 95% CI : 60 to 70). A noninferiority margin of 10.5 % of the 95% CI for the SVR12 of the new regimen established 60% as the noninferiority threshold; the superiority threshold was 70%.
- Analyses by mITT, power > 90%
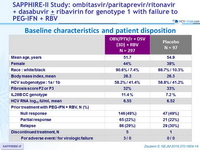
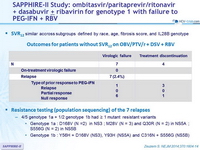
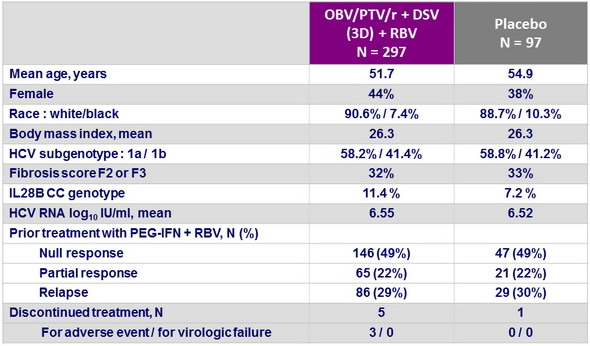
Baseline characteristics and patient disposition
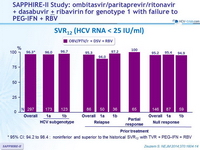
SVR12 (HCV RNA < 25 IU/ml)
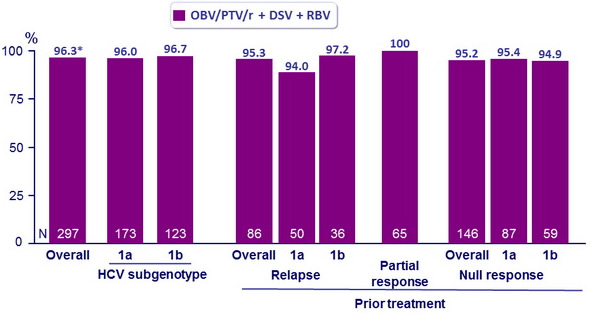
* 95% CI: 94.2 to 98.4 : noninferior and superior to the historical SVR12 with TVR + PEG-IFN + RBV
- SVR12 similar accross subgroups defined by race, age, fibrosis score, and IL28B genotype
Outcomes for patients without SVR12 on OBV/PTV/r + DSV + RBV
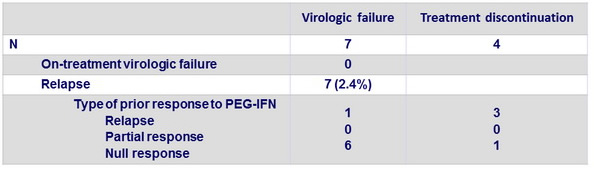
Resistance testing (population sequencing) of the 7 relapses
- 4/5 genotype 1a + 1/2 genotype 1b had ≥ 1 mutant resistant variants
- Genotype 1a : D168V (N =2) in NS3 ; M28V (N = 3) and Q30R (N = 2) in NS5A ; S556G (N = 2) in NS5B
- Genotype 1b : Y56H + D168V (NS3), Y93H (NS5A) and C316N + S556G (NS5B)
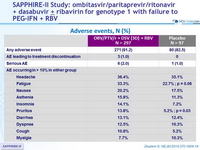
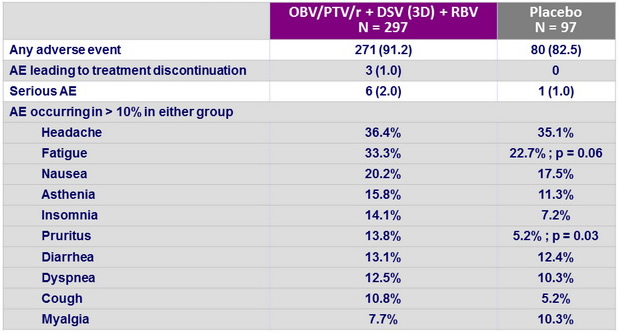
Adverse events, N (%)
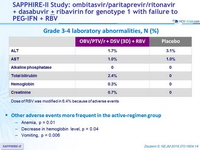
Grade 3-4 laboratory abnormalities, N (%)
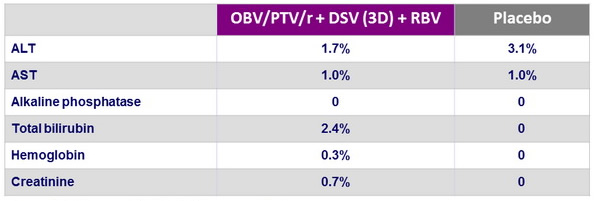
Dose of RBV was modified in 6.4% because of adverse events
Other adverse events more frequent in the active-regimen group
- Anemia, p = 0.01
- Decrease in hemoglobin level, p = 0.04
- Vomiting, p = 0.006
Summary
- Rates of response to a 12-week interferon-free combination regimen of ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin, were more than 95% among previously treated patients with HCV genotype 1 infection, including patients with a prior null response.
- SVR12 was non inferior and superior to the historical control rate with telaprevir plus PEG-IFN + RBV in a similar patient population
- SVR12 was similar in patients with HCV genotype 1a or 1b infection, and in various subgroups (age, sex, fibrosis, IL28B)
- Tolerability was good, with
- 1% of patients discontinuing for AE
- Pruritus, anemia and vomiting more frequent in active group
- Low incidence of grade 3-4 bilirubin elevation
- In conclusion, an all-oral combination regimen of OBV/PTV/r + DSV + RBV resulted in SVR12 > 95%, regardless of HCV genotype (1a or 1b) and with low rates of treatment discontinuation, in previously treated patients with HCV genotype 1 infection and no cirrhosis,, including those with a prior null response