ATOMIC Study: SOF + PEG-IFNα-2a + RBV for HCV genotypes 1, 4, 6
Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicenter phase 2 trial
Kowdley KV. Lancet 2013;381:2100-7
Anti-HCV
Sofosbuvir
PEG-IFNα 2a
Ribavirin
Sofosbuvir
PEG-IFNα 2a
Ribavirin
Genotype
1
1a
1b
1
1a
1b
Treatment history
Naive
Naive
Cirrhosis
No
No
Design
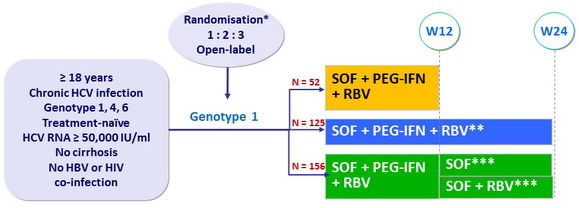
* Randomisation was stratified on IL28 genotype (CC or non-CC) and HCV RNA (< or = 800,000 IU/ml)
** Genotypes 4 and 6 received SOF + PEG-IFN + RBV for 24 weeks
*** Randomisation to extension phase only if HCV RNA < 15 IU/ml at W4
- SOF : 400 mg qd ; PEG-IFNα-2a : 180 µg SC once weekly
- RBV weight based (bid dosing) : 1000 mg/day if < 75 kg or 1200 mg/day if = 75 kg
Objective
-
SVR24 by ITT-analysis, detection of a 30% or 25% difference between two treatment groups, 2-sided significance level of 5%, 90% power
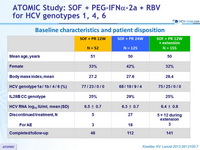
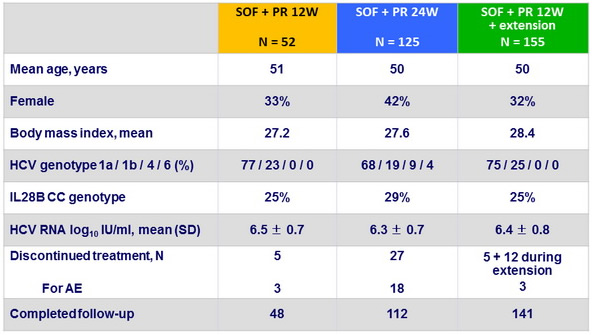
Baseline characteristics and patient disposition
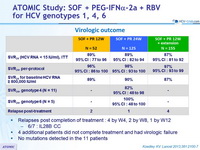
Virologic outcome
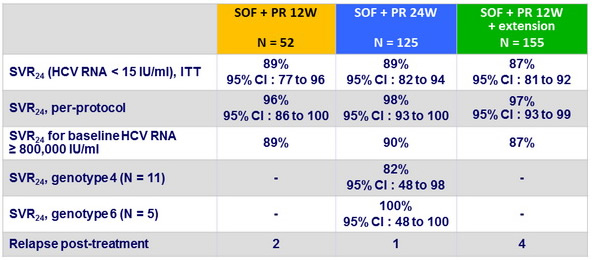
- Relapses post completion of treatment : 4 by W4, 2 by W8, 1 by W12
- 6/7 : IL28B CC
- 4 additional patients did not complete treatment and had virologic failure
- No mutations detected in the 11 patients
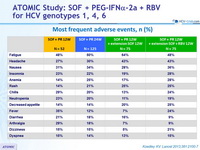
Most frequent adverse events, n (%)
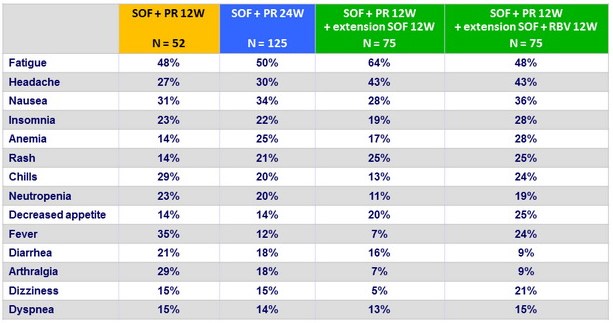
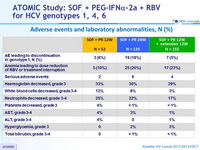
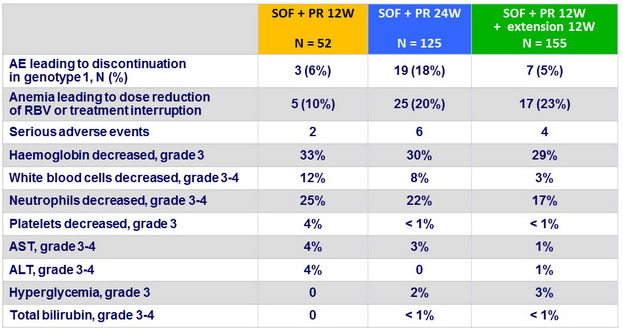
Adverse events and laboratory abnormalities, N (%)
Summary
- 12 week SOF-based regimen is effective for patients with HCV genotypes 1, 4, and 6
- SOF is well tolerated
- There is no additional benefit of extending SOF treatment beyond 12 weeks
- Furthermore, patients in the groups receiving longer durations of PEG-IFN + RBV generally had higher rates of adverse effects without an increase in efficacy
- The uniformly high rates of SVR24 with SOF plus PEG-IFN + RBV also suggest that there would be no need to tailor either the treatment duration or regimen to individual patients on the basis of early response or baseline characteristics
- Limitation : study excluded patients with cirrhosis and those who failed to PI-based treatment