VALENCE Study: SOF + RBV for HCV genotypes 2 and 3
Sofosbuvir and Ribavirin in HCV Genotypes 2 and 3
Zeuzem S. NEJM 2014;370:1993-2001
Anti-HCV
Sofosbuvir
Ribavirin
Sofosbuvir
Ribavirin
Genotype
2
3
2
3
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
Design
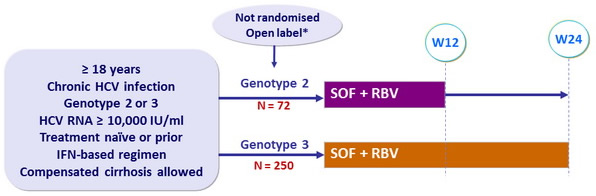
* Initially randomised to 12 weeks SOF + RBV vs placebo (4:1), the study was amended with
unblinding, discontinuation of placebo group and extension to 24 weeks of therapy for genotype 3
- SOF : 400 mg qd
- RBV (bid dosing) : 1000 mg/day if < 75 kg or 1200 mg/day if ≥ 75 kg
Objective
- SVR12 with 95% CI, descriptive analysis
- Multivariate analysis to identify predictors of SVR12
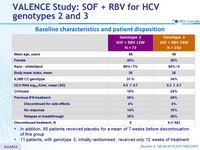
Baseline characteristics and patient disposition
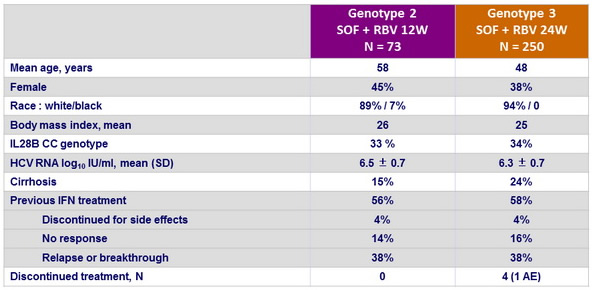
- In addition, 85 patients received placebo for a mean of 7 weeks before discontinuation of this group
- 11 patients, with genotype 3, initially randomised, received only 12 weeks of treatment
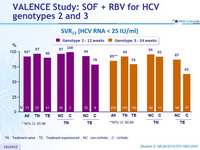
SVR12 (HCV RNA < 25 IU/ml), % (95% CI)
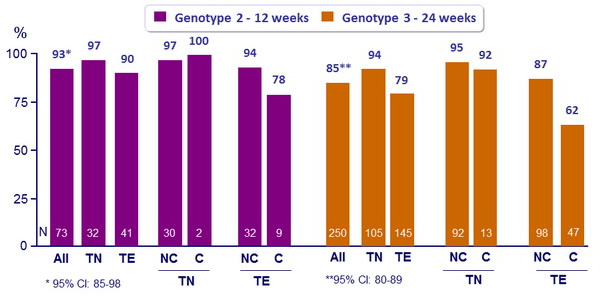
TN : Treatment-naïve ; TE : Treatment-experienced ; NC : non-cirrhotic ; C : cirrhotic
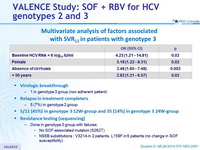
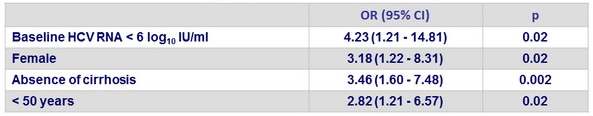
Multivariate analysis of factors associated with SVR12 in patients with genotype 3
Virologic breakthrough
- 1 in genotype 3 group (non adherent patient)
Relapse in treatment completers
- 5 (7%) in genotype 2 group
- 5/11 (45%) in genotype 3 12W-group and 35 (14%) in genotype 3 24W-group
Resistance testing (sequencing)
- Done in genotype 3 group with failures:
- No SOF-associated mutation (S282T)
- NS5B substitutions : V321A in 2 patients, L159F in 6 patients (no change in SOF susceptibility)
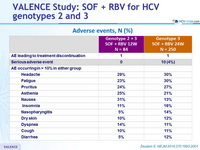
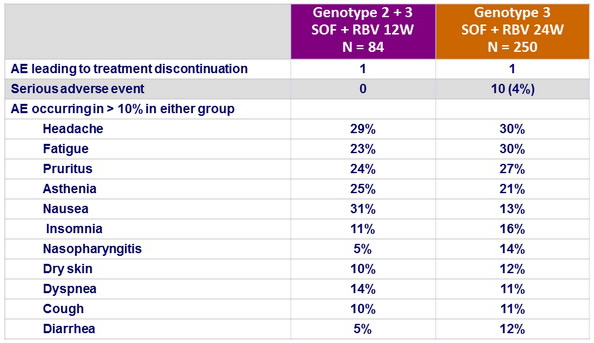
Adverse events, N (%)
Summary
- The oral SOF + RBV regimen resulted in high rates of SVR12 both in genotype 2 and genotype 3 infection
- High rates of response and low rates of relapse in all genotype 2 sub-groups with 12 weeks of SOF + RBV
- For genotype 3, SOF + RBV 24 weeks was associated with higher SVR12 than reported with the same regimen for 12 weeks or 16 weeks
- Identification of 4 possible predictors of SVR12 among patients with genotype 3 infection: female sex, absence of cirrhosis, younger age, and a low viral load at baseline
- Severity and frequency of adverse events did not increase with longer duration of SOF + RBV treatment
- SOF + RBV has a high barrier to resistance
- Limitations
- Descriptive study
- Few number of patients with genotype 2