COMMAND-1 Study: daclatasvir + PEG-IFN + RBV for genotype 1 or 4
Daclatasvir plus peginterferon alfa and ribavirin for treatment-naive chronic hepatitis C genotype 1 or 4 infection: a randomised study
Hezode C. Gut 2015;64:948-56
Anti-HCV
Daclatasvir
PEG-IFNα 2a
Ribavirin
Daclatasvir
PEG-IFNα 2a
Ribavirin
Genotype
1
1a
1b
4
1
1a
1b
4
Treatment history
Naive
Naive
Cirrhosis
Yes
Yes
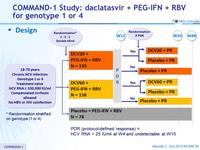
Design
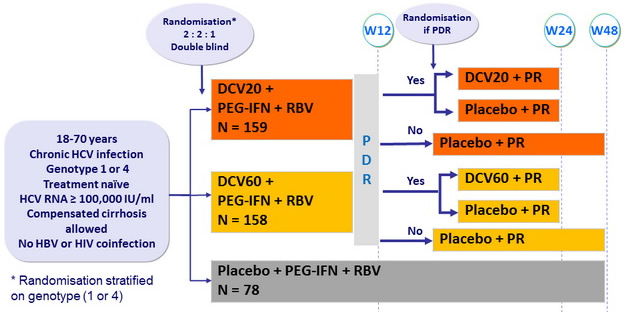
PDR (protocol-defined response) = HCV RNA < 25 IU/ml at W4 and undetectable at W10
Dosage of drugs
- DCV : 20 mg or 60 mg qd or matching placebo
- PEG-IFNα -2a : 180 m g SC once weekly
- RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or = 75 kg)
Co-primary endpoints :
- % of genotype 1 with eRVR (undetectable HCV RNA at W4 and W12) and SVR 24 (undetectable HCV RNA) by m-ITT analysis
Resistance analyses :
- all baseline samples and on-treatment or follow-up samples with HCV RNA = 1000 IU/ml
Objectives
- eRVR of one of DCV dose superior to placebo, in genotype 1 (lower bound of the 80% CI for the difference > 35%, power of 90%)
- SVR 24 of one of DCV dose superior to placebo, in genotype 1 (lower bound of the 80% CI for the difference > 0%, power of 82%)
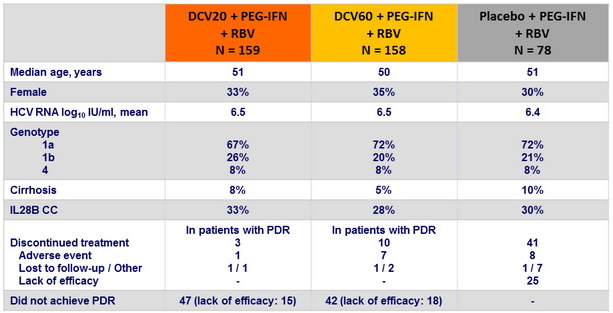
Baseline characteristics and patient disposition
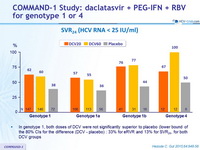
SVR 24 (HCV RNA < 25 IU/ml)
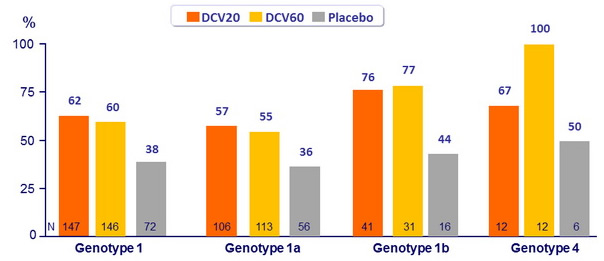
- In genotype 1, both doses of DCV were not significantly superior to placebo (lower bound of the 80% CIs for the difference (DCV - placebo) : 33% for eRVR and 13% for SVR 24 , for both DCV groups
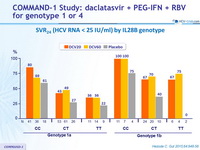
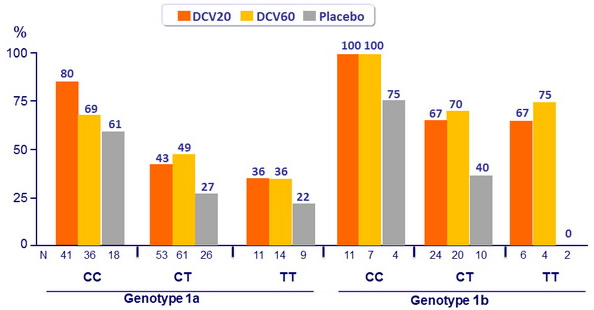
SVR 24 (HCV RNA < 25 IU/ml) by IL28B genotype
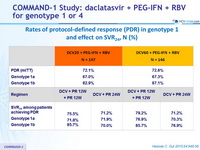
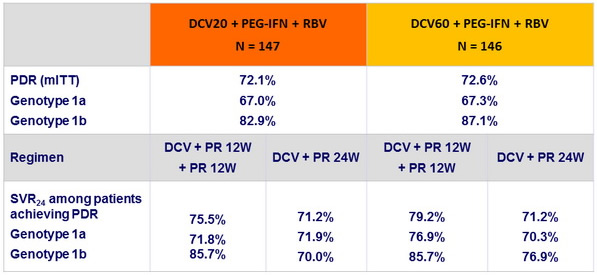
Rates of protocol-defined response (PDR) in genotype 1 and effect on SVR 24, N (%)
Treatment failures in genotype 1, N (%)
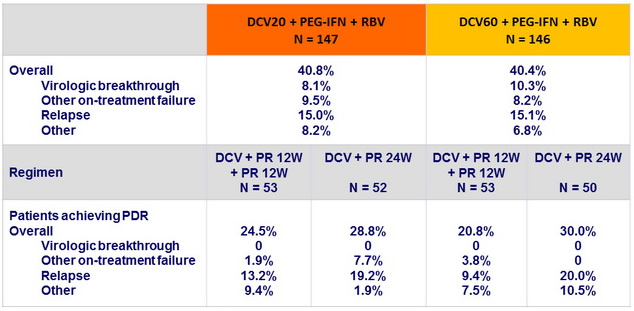
Virologic breakthrough was more frequent in genotype 1a
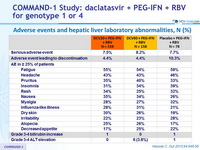
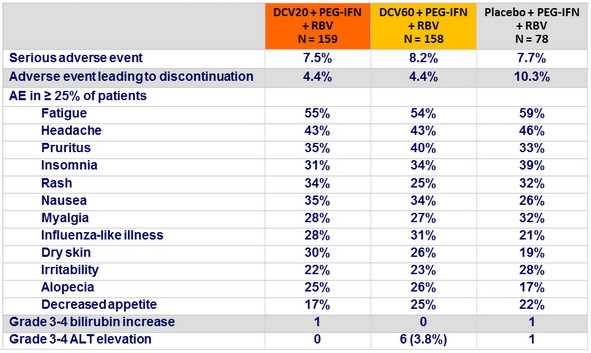
Adverse events and hepatic liver laboratory abnormalities, N (%)
Resistance analysis
- Genotype 1
- At baseline : NS5A polymorphisms associated with DCV resistance (L31M/V and/or Y93H/N/S) detected in 12 genotype 1a patients and 10 genotype 1b patients
- 8/12 genotype 1a (L31M/V and Y93H/N/S) and 2/10 genotype 1b (Y93H) failed to achieve SVR 24
- At virologic failure , DCV-associated resistant variants i n all patients ; most frequent :
- Q30 in genotype 1a
- L31I/M/V and Y93H in genotype 1b
- At baseline : NS5A polymorphisms associated with DCV resistance (L31M/V and/or Y93H/N/S) detected in 12 genotype 1a patients and 10 genotype 1b patients
- Genotype 4
- 4 virologic failures , sequencing available in 3 patients ; emergence of L28M + L30H in 1 patient, and L28M + L30S in 2 patients
Summary
- In this phase IIb study, the combination of daclatasvir + PEG-IFN + RBV was generally well tolerated and achieved higher SVR 24 rates compared with placebo + PEG-IFN + RBV among patients infected with HCV genotype 1 or 4
- Genotype 1a patients had lower rates of SVR 24 and higher rates of virologic failure compared with genotype 1b patients
- Although sample size was small, SVR 24 was 100% with DCV 60 mg + PEG-IFN + RBV in genotype 4
- IL28B genotype predicted response, with higher rates of SVR 24 observed among patients with a CC genotype
- Dose of 60 mg QD of DCV selected for Phase III studies