C-SALVAGE Study: grazoprevir + elbasvir + RBV in genotype 1 with failure to PI-based regimen
Grazoprevir and elbasvir plus ribavirin for chronic HCV genotype-1 infection after failure of combination therapy containing a direct-acting antiviral agent
Forns X. J Hepatology 2015; 63: 564-72
Anti-HCV
Grazoprevir
Elbasvir
Ribavirin
Grazoprevir
Elbasvir
Ribavirin
Genotype
1
1a
1b
1
1a
1b
Treatment history
PI (NS3)-experienced
PI (NS3)-experienced
Cirrhosis
Yes
No
Yes
No
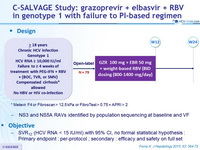
Design
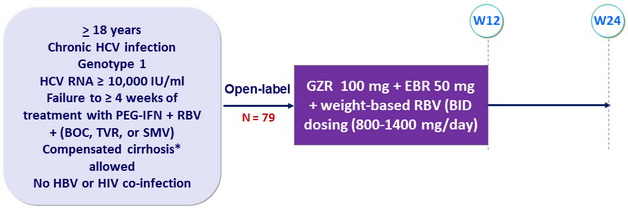
* Metavir F4 or Fibroscan > 12.5 kPa or FibroTest > 0.75 + APRI > 2
- NS3 and NS5A RAVs identified by population sequencing at baseline and VF
Objective
- SVR12 (HCV RNA < 15 IU/ml) with 95% CI, no formal statistical hypothesis : Primary endpoint : per-protocol ; secondary : efficacy and safety on full set
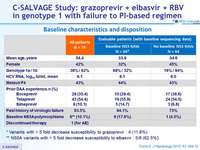
Baseline characteristics
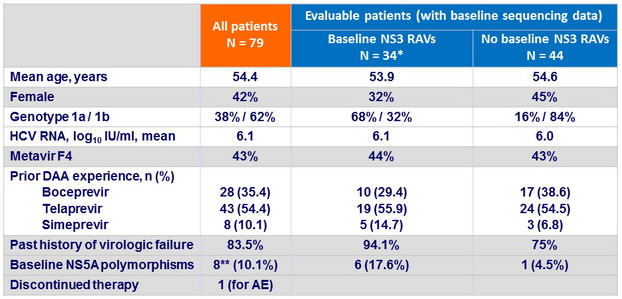
* Variants with > 5 fold decrease susceptibility to grazoprevir : 4 (11.8%)
** NS5A variants with > 5 fold decrease susceptibility to elbasvir : 5/8 (62.5% )
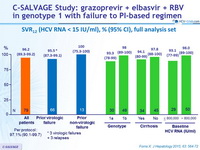
SVR12 (HCV RNA < 15 IU/ml), % (95% CI), full analysis set
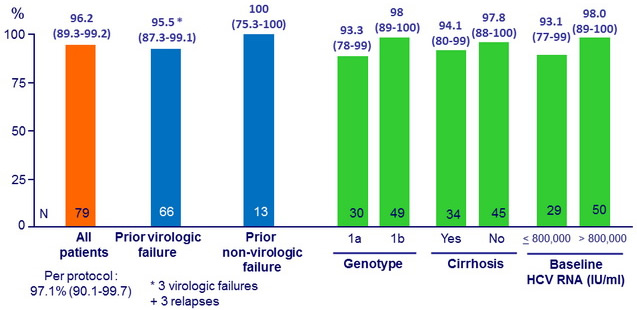
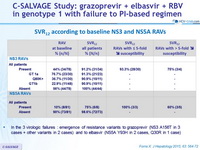
SVR12 according to baseline NS3 and NS5A RAVs
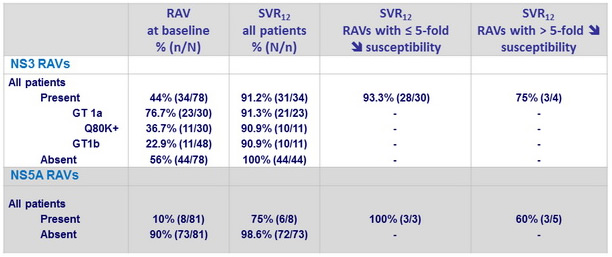
- In the 3 virologic failures : emergence of resistance variants to grazoprevir (NS3 A156T in 3 cases + other variants in 2 cases) and to elbasvir (NS5A Y93H in 2 cases, Q30R in 1 case)
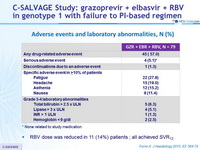
Adverse events and laboratory abnormalities, N (%)
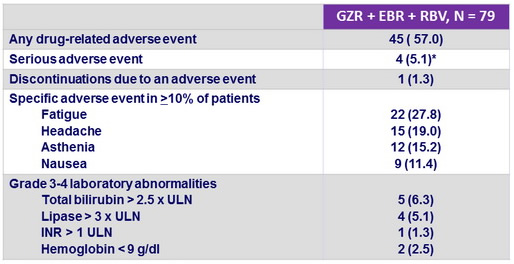
* None related to study medication
- RBV dose was reduced in 11 (14%) patients ; all achieved SVR12
Summary
- GZR + EBR + RBV for 12 weeks resulted in SVR12 in 76/79 patients (96.2%) who had failed previous PEG-IFN + RBV plus an earlier-generation PI
- SVR12 was attained in 63/66 (95.5%) patients with a history of VF
- SVR12 was attained in 31/34 (91.2%) patients harboring baseline NS3 RAVs conferring decreased susceptibility to 1st-generation PIs
- GZR + EBR + RBV was generally well tolerated
- Only 1 patient stopped study treatment (adverse event unrelated to study treatment)
- No serious drug-related adverse event