GIFT-I Study: ombitasvir/paritaprevir/ritonavir for genotype 1b japanese patients
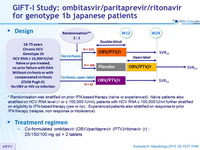
Randomized Phase 3 Trial of Ombitasvir/Paritaprevir/Ritonavir for HCV Genotype 1b infected Japanese Patients With or Without Cirrhosis
Kumada H. Hepatology 2015; 62:1037-1046
Anti-HCV
Ombitasvir
Paritaprevir/ritonavir
Ombitasvir
Paritaprevir/ritonavir
Genotype
1b
1b
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
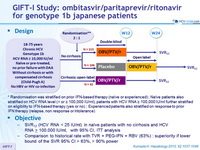
Design
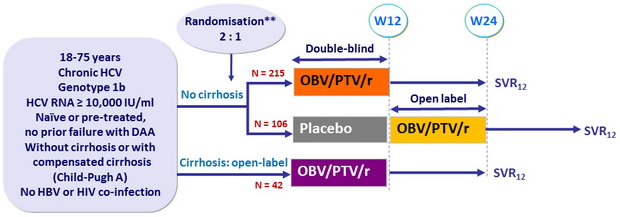
*Randomisation was stratified on prior IFN-based therapy (naïve or experienced) ; Naïve patients also stratified on HCV RNA level (< or = 100,000 IU/ml), patients with HCV RNA = 100,000 IU/ml further stratified on eligibility to IFN-based therapy (yes or no) ; Experienced patients also stratified on response to prior IFN therapy (relapse, non response or intolerance)
Treatment regimen
- Co-formulated ombitasvir (OBV)/ paritaprevir (PTV)/ ritonavir (r) : 25/150/100 mg qd = 2 tablets
Objective
- SVR12 (HCV RNA < 25 IU/ml) in naïve patients with no cirrhosis and HCV RNA > 100.000 IU/ml, with 95% CI, ITT analysis
- Comparison to historical rate with TVR + PEG-IFN + RBV (63%) : superiority if lower bound of the SVR 95% CI > 63%, > 90% power
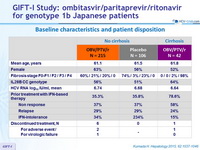
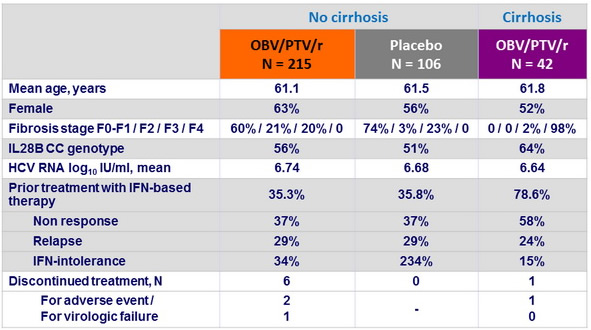
Baseline characteristics and patient disposition
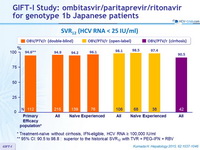
SVR12 (HCV RNA < 25 IU /ml)
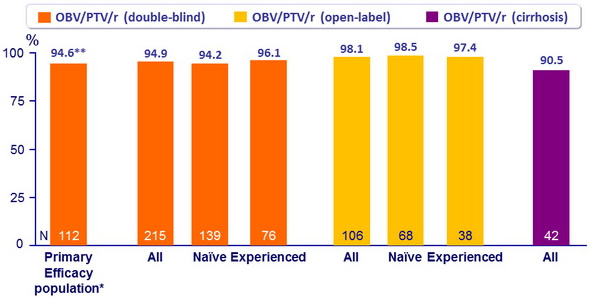
* Treatment-naïve without cirrhosis, IFN-eligible, HCV RNA = 100,000 IU/ml
** 95% CI: 90.5 to 98.8 : superior to the historical SVR12 with TVR + PEG-IFN + RBV
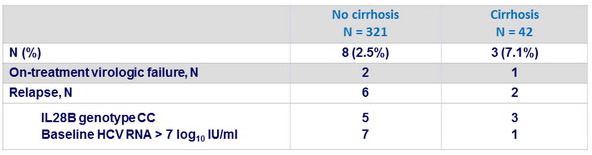
Patients without SVR12 on OBV/PTV/r
Resistance testing (population sequencing)
- A t baseline, NS3 resistance-associated variants in 1%, NS5A RAVs in 38%
- At failure, 10/11 patients had both NS3 and NS5A RAVs
- NS3 : D168V alone or + Y56H, N = 8 ; D168A + Y56H, N = 2
- NS5A : Y93H alone or + L28M, R30Q, L31M, L31V and/or P58S, N = 10 (in 8/10, Y93H present at baseline) ; L31F, N = 1
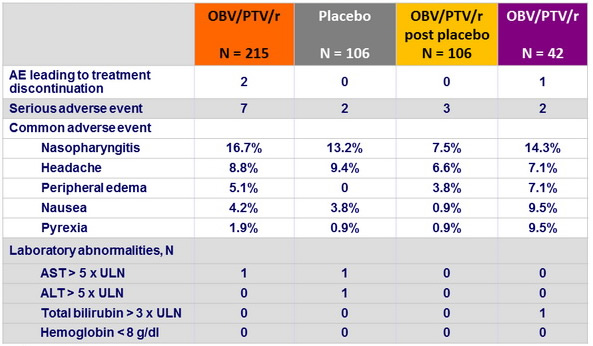
Adverse events and = grade 3 laboratory abnormalities, n (%)
Summary
- In this phase III trial in Japanese patients with HCV genotype 1b infection with or without cirrhosis, high SVR12 rates were achieved with 12 weeks of the IFN-free and RBV -free regimen of OBV/PTV/r
- Rate of virologic failure was low (3%) with RAVs observed in both NS3 and NS5A in 10 of 11 patients with virologic failure, including 8 patients with pre-existing NS5A RAV Y93H
- Y93H is present in 8.2% to 25% Japanese with HCV genotype 1b
- In this study, Y93H was present at baseline in 14% of patients and SVR12 was 83% in patients with this RAV at baseline
- Among treatment-emergent adverse events, only peripheral edema occurred more frequently with OBV/PTV/r than with placebo
- All patients who experienced peripheral edema were also taking concurrent calcium channel blockers, with a risk related to the dose of the CCB