ION-4 Study: LDV/SOF in HIV co-infection
Ledipasvir and Sofosbuvir for HCV in Patients Coinfected with HIV-1
Naggie S. N Engl J Med. 2015 Aug 20;373(8):705-13
Anti-HCV
Ledipasvir
Sofosbuvir
Ledipasvir
Sofosbuvir
Genotype
1
1a
1b
1
1a
1b
Special population
HIV co-infection
HIV co-infection
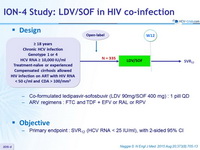
Design
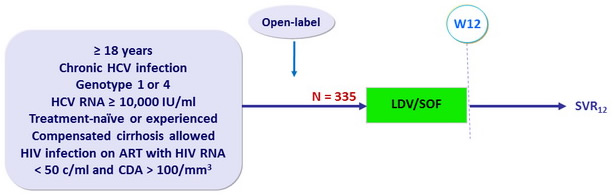
- Co-formulated ledipasvir-sofosbuvir (LDV 90mg/SOF 400 mg) : 1 pill QD
- ARV regimens : FTC and TDF + EFV or RAL or RPV
Objective
- Primary endpoint : SVR12 (HCV RNA < 25 IU/ml), with 2-sided 95% CI
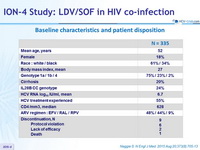
Baseline characteristics and patient disposition

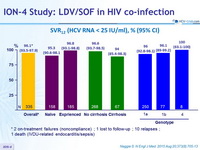
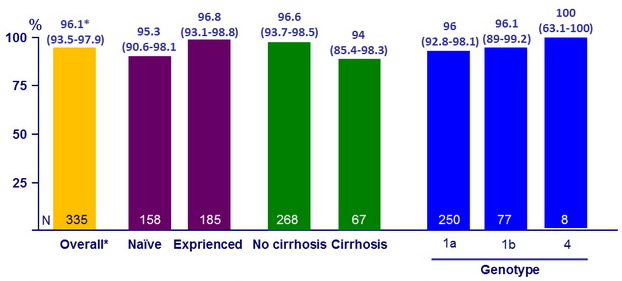
SVR12 (HCV RNA < 25 IU/ml), % (95% CI)
* 2 on-treatment failures (noncompliance) ; 1 lost to follow-up ; 10 relapses ;
1 death (IVDU-related endocarditis/sepsis)
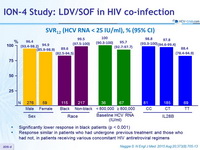
SVR12 (HCV RNA < 25 IU/ml), % (95% CI)
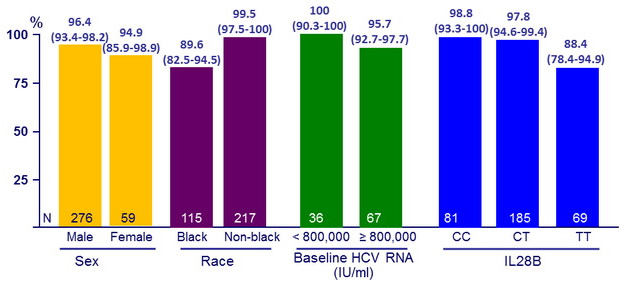
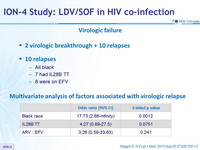
- Significantly lower response in black patients (p < 0.001)
- Response similar in patients who had undergone previous treatment and those who had not, in patients receiving various concomitant HIV antiretroviral regimens
Virologic failure
- 2 virologic breakthrough + 10 relapses
- 10 relapses
- All black
- 7 had IL28B TT
- 8 were on EFV
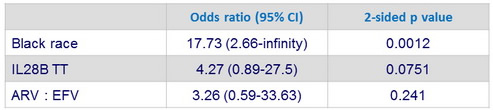
Multivariate analysis of factors associated with virologic relapse
Virologic resistance testing
- Patients with genotype 1 : deep sequencing of NS5A at baseline : 59/325 (18% ) patients with NS5A variants (RAVs)
- 55/59 (93%) achieved SVR12
- 258/266 (97%) with no baseline NS5A RAVs achieved SVR12
- 2 patients with virologic breakthrough : no baseline NS5A RAVs but emergence of NS5A RAVs at failure
- 10 relapses: NS5A RAVs at baseline in 4, and in 8 at the time of relapse
- No NS5B S282T in any patient at baseline or virologic failure
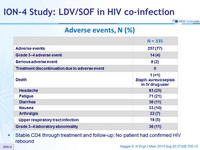
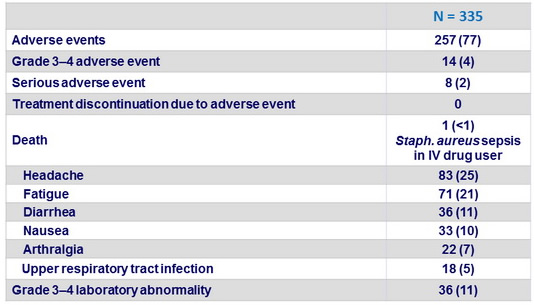
Adverse events, N (%)
- Stable CD4 through treatment and follow-up; No patient had confirmed HIV rebound
Summary
- In this Phase III study of 335 HIV/HCV- coinfected patients, 96% achieved SVR12 after 12 weeks of a once-daily, single-tablet regimen of LDV/SOF
- Prior HCV treatment status or the presence or absence of cirrhosis did not impact outcome
- In contrast to larger studies among monoinfected patients, a lower response rate was observed among coinfected black patients treated with LDV/SOF ( SVR12 : 90 %)
- LDV/SOF was well tolerated, with no treatment discontinuations due to adverse events and no adverse impact on HIV disease or its treatment