OPTIMIST-2 Study: SMV + SOF for genotype 1 and cirrhosis
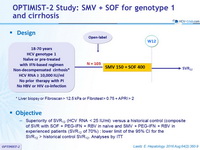
A Phase 3, Open-Label, Single-ARM Study to Evaluate the Efficacy and Safety of 12 Weeks of Simeprevir (SMV) plus Sofosbuvir (SOF) in Treatment-Naïve or -Experienced Patients with Chronic HCV Genotype 1 Infection and Cirrhosis: OPTIMIST-2
Lawitz E. Hepatology. 2016 Aug;64(2):360-9
Anti-HCV
Sofosbuvir
Simeprevir
Sofosbuvir
Simeprevir
Genotype
1
1a
1b
1
1a
1b
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
Yes
Design
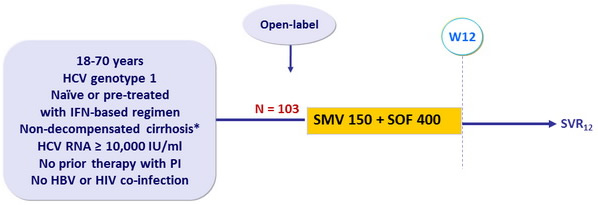
* Liver biopsy or Fibroscan > 12.5 kPa or Fibrotest > 0.75 + APRI > 2
Objective
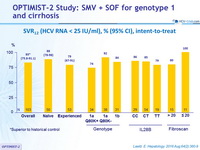
- Superiority of SVR12 (HCV RNA < 25 IU/ml) versus a historical control (composite of SVR with SOF + PEG-IFN + RBV in naïve and SMV + PEG-IFN + RBV in experienced patients (SVR12 of 70%) : lower limit of the 95% CI for the SVR12 > historical control SVR12. Analyses by ITT
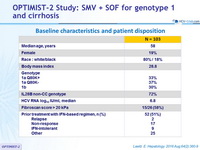
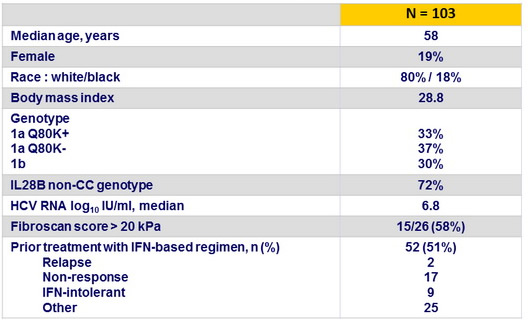
Baseline characteristics and patient disposition
SVR12 (HCV RNA < 25 IU/ml), % (95% CI), intent-to-treat
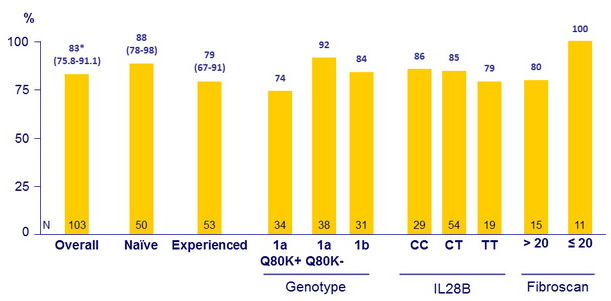
* Superior to historical control
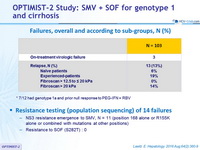
Failures, overall and according to sub-groups, N (%)

* 7/12 had genotype 1a and prior null response to PEG-IFN + RBV
Resistance testing (population sequencing) of 14 failures
- NS3 resistance emergence to SMV, N = 11 (position 168 alone or R155K alone or combined with mutations at other positions)
- Resistance to SOF (S282T) : 0
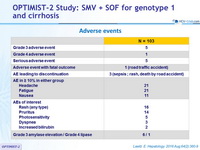
Adverse events
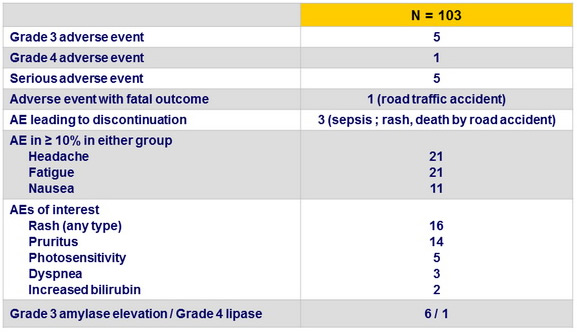
Summary
- The combination of SMV + SOF for 12 weeks
- demonstrated superiority in SVR12 rates (83%) versus the historical control (70%) in treatment-naïve and -experienced HCV genotype-1 infected patients with cirrhosis
- achieved SVR12 rates higher for genotype 1a without Q80K (92%) versus those with Q80K (74%)
- SVR12 was = 85% for patients with IL28B CC or CT, for treatment-naïve patients and for patients with platelet count = 90,000/mm 3
- SVR12 was = 94% for cirrhotic patients with albumin = 4 g/dl and FibroScan score >12.5 to = 20 kPa
- Viral relapse was the primary reason for not achieving SVR12
- Patient-reported outcomes scores significantly improved from baseline, as observed by the Week 12 follow-up visit coincident with the SVR12 assessment
- SMV + SOF for 12 weeks was safe and well tolerated; most adverse events were Grade 1 or 2