NIAID SYNERGY GT4 Study: LDV/SOF in genotype 4
Ledipasvir and sofosbuvir for hepatitis C genotype 4: a proof-of-concept, single-centre, open-label phase 2a cohort study
Kohli A. Lancet Infect Dis. 2015 Sep;15(9):1049-54
Anti-HCV
Ledipasvir
Sofosbuvir
Ledipasvir
Sofosbuvir
Genotype
4
4
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
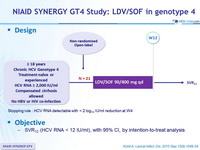
Design
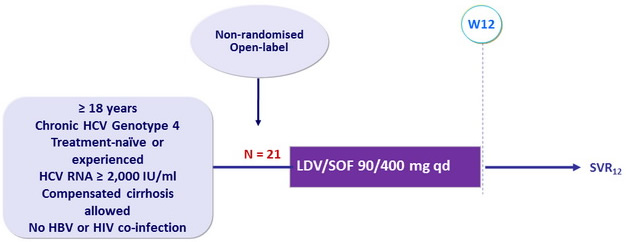
Stopping rule : HCV RNA detectable with < 2 log 10 IU/ml reduction at W4
Objective
- SVR12 (HCV RNA < 12 IU /ml) , with 95% CI, by intention-to-treat analysis
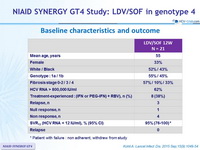
Baseline characteristics and outcome
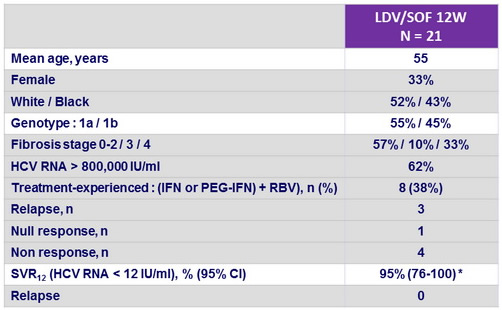
* Patient with failure : non adherent, withdrew from study
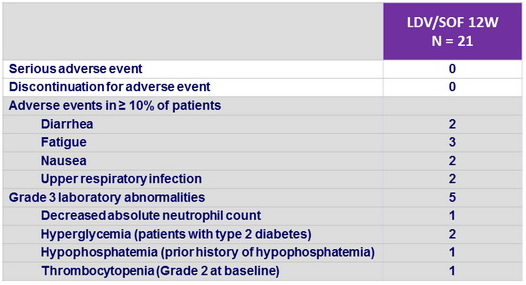
Adverse events, N
Summary
- Patients with chronic HCV genotype 4 infection were successfully treated with a 12 week course of LDV/SOF
- SVR12 was 100 % for patients who received all 12 weeks of study drugs, irrespective of previous treatment status and underlying liver fibrosis
- All patients on therapy had HCV RNA below the lower limit of quantification by W4
- This is the first report of a single-pill, all-oral, interferon-free, ribavirin-free treatment for patients with HCV genotype 4
- Limitations
- Small sample size
- No randomisation