GS-9451/GS-9256 Failure Study: SOF + PEG-IFN + RBV for genotype 1 and prior failure to DAA
Sofosbuvir Plus Pegylated Interferon and Ribavirin in Patients With Genotype 1 Hepatitis C Virus in Whom Previous Therapy With Direct-Acting Antivirals Has Failed
Pol S. Hepatology 2015; 62:129-34
Anti-HCV
Sofosbuvir
Ribavirin
PEG-IFNα 2a
Sofosbuvir
Ribavirin
PEG-IFNα 2a
Genotype
Treatment history
IFN-Experienced
PI (NS3)-experienced
IFN-Experienced
PI (NS3)-experienced
Cirrhosis
No
No
Design
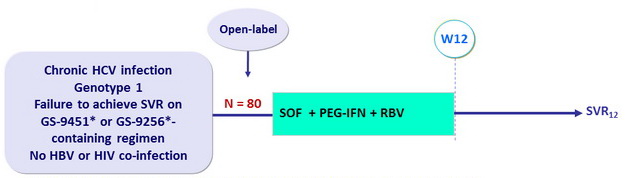
* GS-9451 ( vedroprevir ) : 1st generation PI ; GS-9256 : 1st generation PI
- SOF 400 mg : 1 pill qd
- PEG-IFNα-2a 180 µg SC once weekly
- RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or = 75 kg)
Objective
- Primary endpoint : SVR12 (HCV RNA < 25 IU /ml) by intention to treat, with 2-sided 95% CI, no statistical hypothesis
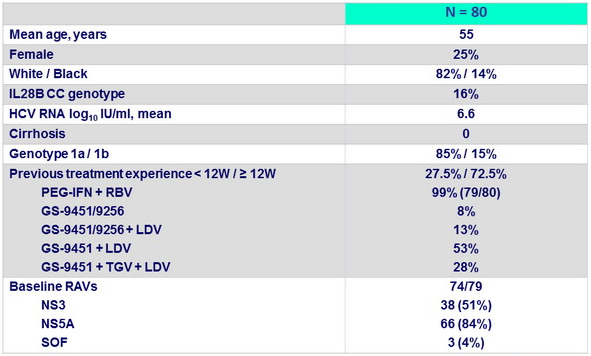
Baseline characteristics
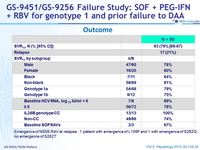
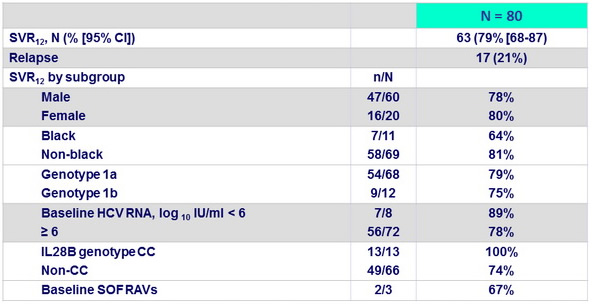
Outcome
Emergence of NS5B RAV at relapse : 1 patient with emergence of L159F and 1 with emergence of S282G ; no emergence of S282T
Adverse events
- Serious adverse event : 1 (dysphagia , unrelated)
- Discontinuation due to adverse event : 3 (PEG-IFN in 2, RBV in 1)
- Most common adverse events :
- Fatigue (43%)
- Headache (35%)
- Nausea (24%)
- Neutropenia (23%)
- Influenza-like illness (19%)
- Myalgia (15%)
- Pruritus (15%)
- Rash (15%)
- Grade 3 / grade 4 laboratory abnormalities : 41% / 10%
- Mean decrease in hemoglobin : -2.8 g/dl
Summary
- In this open-label study, 12 weeks of treatment with SOF + PEG- IFN + RBV resulted in a high rate of SVR12 in patients without cirrhosis who had not achieved SVR in previous trials involving PEG- IFN/RBV plus a protease inhibitor with and without other DAAs
- Similar SVR12 by baseline patient characteristics
- High efficacy regardless of baseline RAVs : benefit of using a different class (SOF)
- Limitations
- Small sample size
- Uncontrolled design
- Lack of patients with cirrhosis