ASTRAL-4 Study: SOF/VEL in patients with decompensated liver disease
ASTRAL-4 Study: SOF/VEL in patients with decompensated liver disease
Curry MP. N Engl J Med 2015; 373: 2618-28
Anti-HCV
Velpatasvir (GS-5816)
Sofosbuvir
Ribavirin
Velpatasvir (GS-5816)
Sofosbuvir
Ribavirin
Genotype
1a
1b
3
1a
1b
3
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
Yes
Special population
Decompensated liver disease
Decompensated liver disease
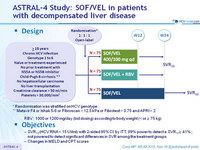
Design
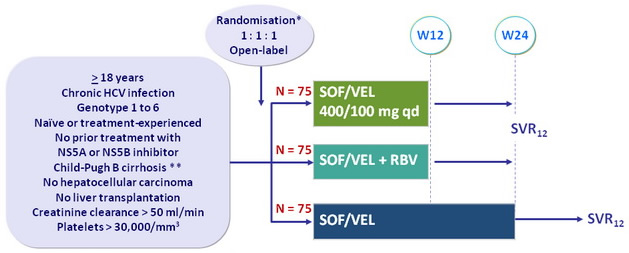
* Randomisation was stratified on HCV ge notype
** Metavir F4 or Ishak 5-6 or Fibroscan > 12.5 kPa or Fibrotest > 0.75 and APRI > 2
- RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or = 75 kg)
Objective
- SVR12 (HCV RNA < 15 UI/ml) with 2-sided 95% CI , by ITT, 99% power to detect a SVR 12 = 41% ; not powered to detect significant differences in SVR among the treatment groups
- Changes in MELD and CPT scores
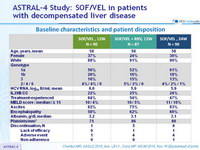
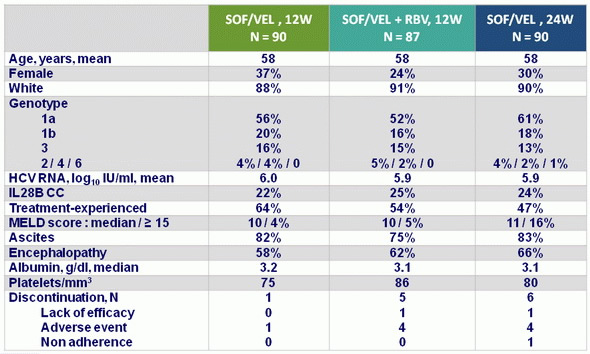
Baseline characteristics and patient disposition
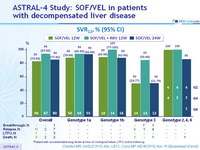
SVR12, % (95% CI)
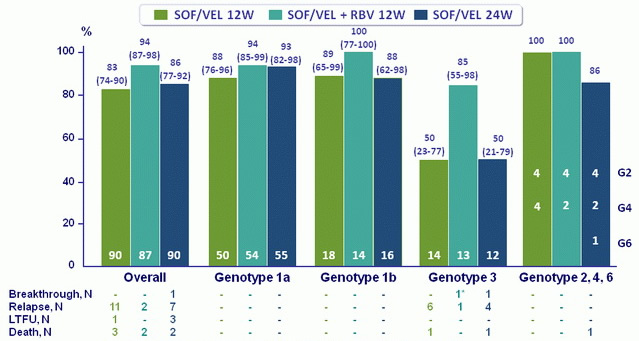
*Patient with non-detectable drug levels at time of virological failure, LTFU, lost to follow-up
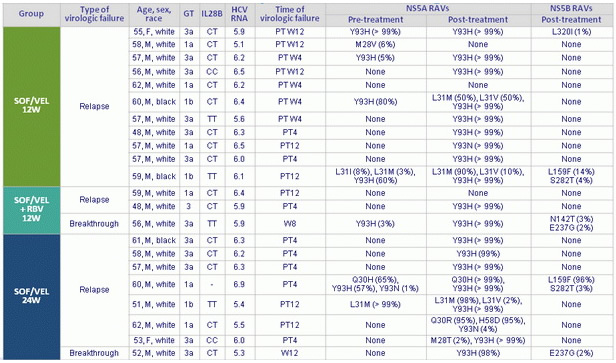
Characteristics of patients with virologic failure
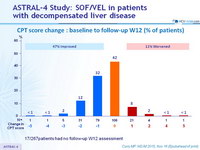
CPT score change : baseline to follow-up W12 (% of patients)
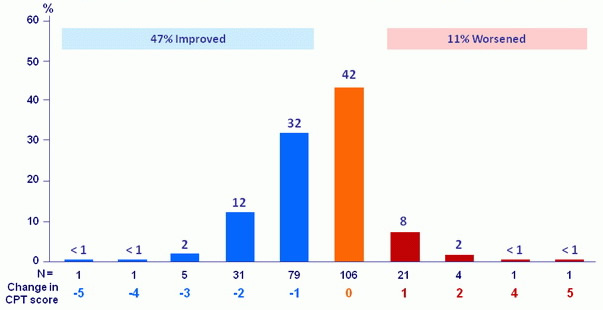
17/267patients had no follow-up W12 assessment
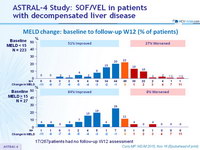
MELD change: baseline to follow-up W12 (% of patients)
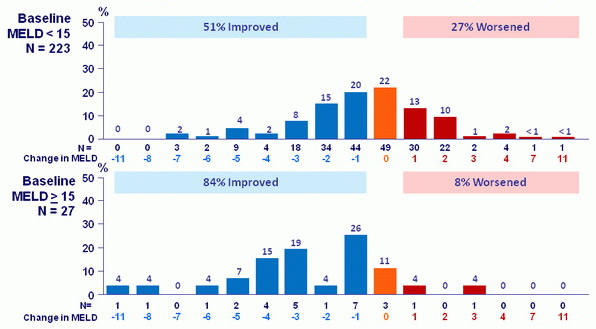
17/267patients had no follow-up W12 assessment
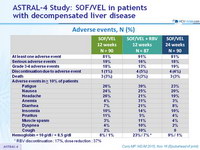
Adverse events, N (%)
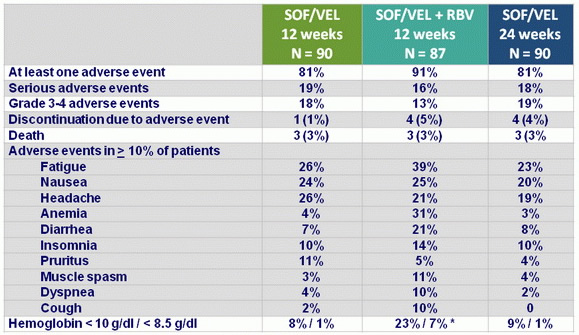
* RBV discontinuation : 17%, dose reduction : 37%
Hematologic abnormalities, N (%)
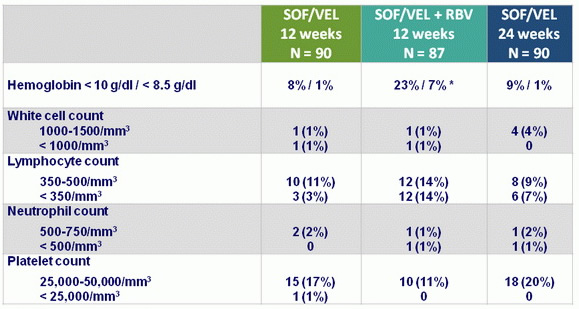
* RBV discontinuation : 17%, dose reduction : 37%
Summary
- Treatment with SOF/VEL for 12 or 24 weeks or SOF/VEL + RBV for 12 weeks resulted in high SVR12 rates in HCV patients with decompensated cirrhosis caused by HCV of all genotypes
- SOF/VEL + RBV resulted in the highest overall SVR12 rates, with the lowest rates of virologic failure in HCV genotype 3 patients
- Treatment was associated with improved MELD and CPT scores largely due to decreased bilirubin and improvement in synthetic function (albumin)
- SOF/VEL for 12 or 24 weeks or SOF/VEL + RBV for 12 weeks was safe and well tolerated, with adverse events consistent with clinical sequelae of advanced liver disease and RBV toxicity
- Limitations
- Study not powered to detect significant differences among the 3 treatment groups
- Only patients with moderate hepatic decompensation were enrolled
- The numbers of patients with HCV genotype 2, 4, or 6 were small
- Limited number of black patients