QUARTZ-I Study: OBV/PTV/r + DSV + SOF ± RBV for HCV genotype 1 after failure of DAA regimens
QUARTZ-I Study: OBV/PTV/r + DSV + SOF ± RBV for HCV genotype 1 after failure of DAA regimens
Poordad F. AASLD 2015, Abs. LB20, EASL 2016, Abs. SAT-156, J Hepatol 2016; 64:S767
Anti-HCV
Paritaprevir/ritonavir
Ombitasvir
Dasabuvir
Sofosbuvir
Ribavirin
Paritaprevir/ritonavir
Ombitasvir
Dasabuvir
Sofosbuvir
Ribavirin
Genotype
1
1a
1
1a
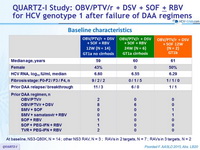
Treatment history
PI (NS3)-experienced
NS5A experienced
PI (NS3)-experienced
NS5A experienced
Cirrhosis
Yes
No
Yes
No
Design
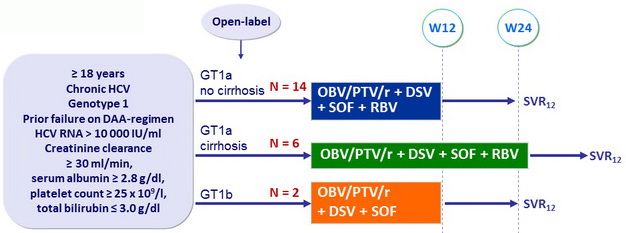
Treatment regimens
- Co-formulated ombitasvir (OBV)/ paritaprevir (PTV)/ rironavir (r): 25/150/100 mg qd = 2 tablets
- Dasabuvir (DSV): 250 mg bid
- RBV: 1000 or 1200 mg/day (bid dosing) according to body weight (< or ≥ 75 kg )
Objective
- SVR12 (HCV RNA < 25 IU/ml), by ITT
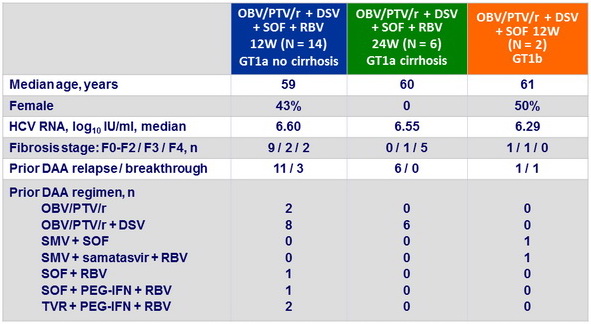
Baseline characteristics
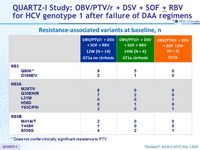
At baseline, NS3-Q80K, N = 14 ; other NS3 RAV, N = 3 ; RAVs in 2 targets, N = 7 ; RAVs in 3 targets, N = 2
Resistance-associated variants at baseline, n
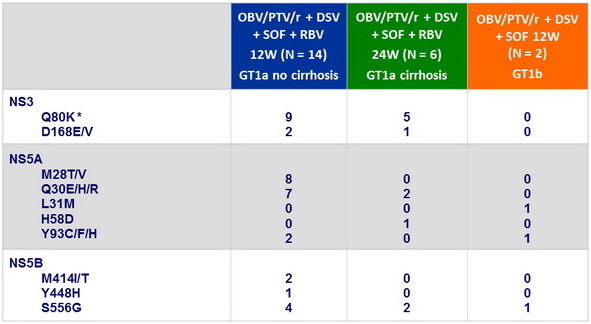
* Does not confer clinically significant resistance to PTV
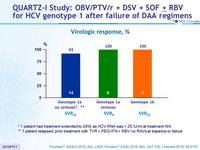
Virologic response, %
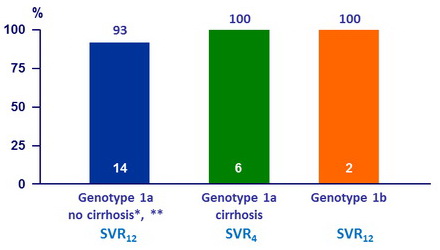
* 1 patient had treatment extended to 24W, as HCV RNA was > 25 IU /ml at treatment W4
** 1 patient relapsed, prior treatment with TVR + PEG-IFN + RBV, no RAVs at baseline or failure
Adverse events, n
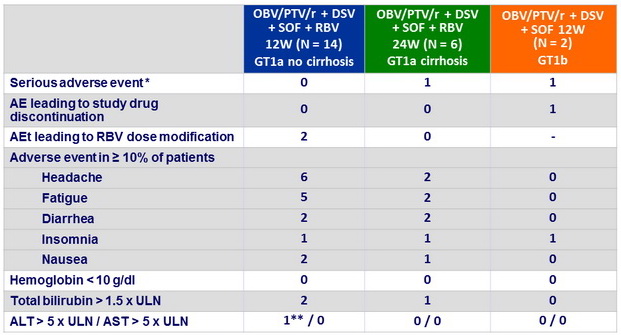
* 1
case of pneumonia, 1 case of cellulitis (both unrelated) ; ** at D15 and D22, resolved by end of treatment
Summary
- A virologic response of 95.2% was achieved with the multi-targeted regimen of OBV/PTV/r + DSV + SOF ± RBV in patients with DAA treatment experience, including those with RAVs at baseline
- SVR12 rate of 93% (14/15) in patients having terminated follow-up
- Treatment was well tolerated with no discontinuations