MALACHITE study: OBV/PTV/r + DSV ± RBV versus telaprevir + PEG-IFN + RBV in genotype 1
Dore G. J Hepatol 2016; 64:19-28
Anti-HCV
Ombitasvir
Paritaprevir/ritonavir
Dasabuvir
Ribavirin
Ombitasvir
Paritaprevir/ritonavir
Dasabuvir
Ribavirin
Genotype
1
1a
1b
1
1a
1b
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
No
No
Design
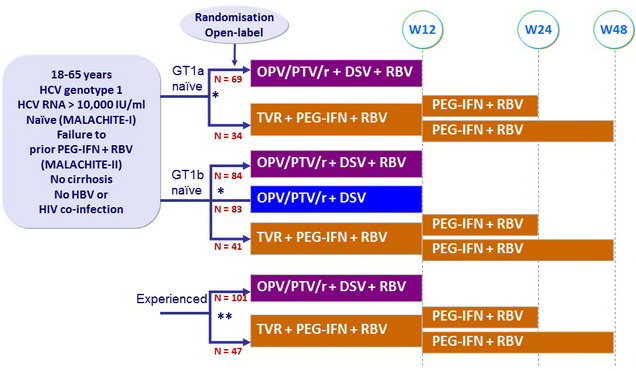
* Randomisation was stratified on IL28B (CC or non-CC)
** Randomisation was stratified
on genotype (1a or 1b) and previous response to PEG-IFN + RBV
(null response, partial response, relapse)
Treatment regimens
- Co-formulated ombitasvir (OBV)/ paritaprevir (PTV)/ rironavir (r ): 25/150/100 mg qd = 2 tablets
- Dasabuvir (DSB) : 250 mg bid
- TVR: 750 mg tid , 8h apart
- PEG -IFNα-2a : 180 m g SC once weekly
- RBV: 1000 or 1200 mg/day (bid dosing) according to body weight (< or = 75 kg )
- In TVR groups, additional 12 or 36 weeks of PEG-IFN + RBV depended on virologic response at treatment W4-12
Primary efficacy endpoint
- MALACHITE-I: Non inferiority of OBV/PTV/r + DSB + RBV compared to TVR + PEG-IFN + RBV in genotype 1a and of OBV/PTV/r + DSB compared to TVR + PEG-IFN + RBV in genotype 1b : SVR12 (HCV RNA < 25 IU/ml) by intention to treat (lower margin of the 2-sided 95% CI for the difference with TVR = - 10.5%)
- MALACHITE-II: % of patients achieving SVR12 between treatment arms using a logistic regression model with treatment arm, baseline log10 HCV RNA level, HCV subgenotype ( 1a, non-1a), and previous PEG-IFN + RBV treatment response (relapse, partial response, null response)
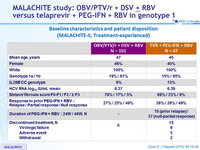
Baseline characteristics and patient disposition (MALACHITE-I, treatment-naïve)
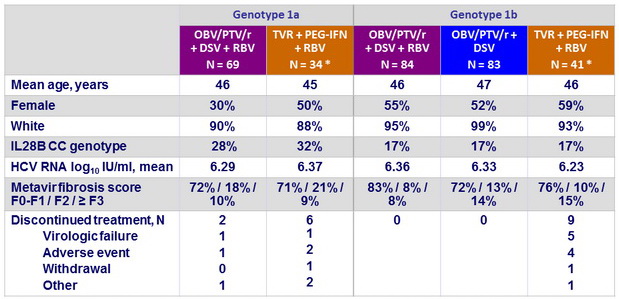
* Among the 75 patients in the TVR groups, 59 received 24 weeks of PEG-IFN + RBV, 16 received 48 weeks
Baseline characteristics and patient disposition (MALACHITE-II, Treatment-experienced)
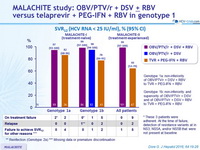
SRV12 (HCV RNA < 25 IU /ml), % (95% CI)
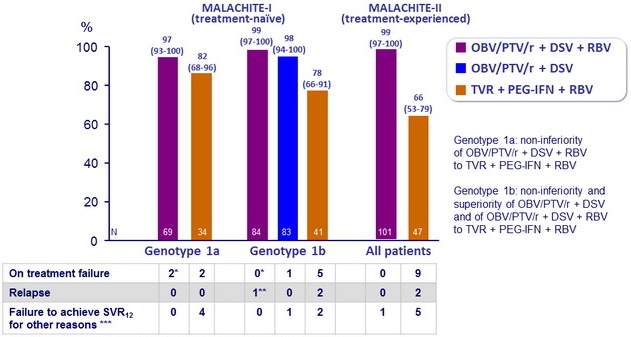
* These 3 patients were adherent. At the time of failure, detection of resistance variants at in NS3, NS5A, and/or NS5B that were not present at baseline
** Reinfection (Genotype 2a)
*** Missing data or premature discontinuation
Adverse events
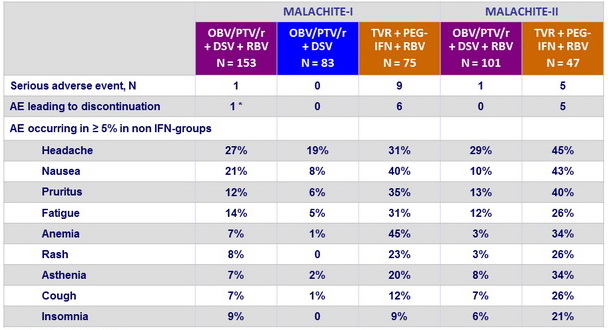
* Hot flush and fatigue
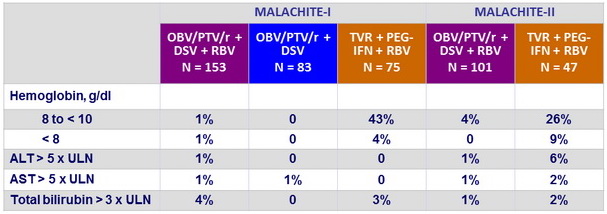
Laboratory abnormalities, %
Patient-reported outcomes
- SF-36v2 self-administered questionnaire: overall, the difference between the 2 regimens in the changes from baseline in Mental Component Summary and Physical Component Summary throughout the treatment and post-treatment periods in both treatment-naïve and treatment-experienced patients favored the OBV/PTV/r + DSV ± RBV regimen
Summary
- This is the first head-to-head study of an all-oral , DAA (OBV/PTV/ r + DSV ± RBV ) and a PEG-IFN - containing (TVR + PEG-IFN + RBV ) regimen that quantitatively compares efficacy and safety benefits in treatment-naïve and treatment -experienced HCV genotype 1 -infected patients
- SVR12 rate was numerically higher with OBV/PTV/ r + DSV + RBV (97-99%) than with TVR + PEG-IFN + RBV (66-82%) regardless of subgenotype or prior treatment status
- Better tolerability of OBV/PTV/r + DSV + RBV
- Higher improvement in mental and physical health in patients receiving OBV/PTV/ r + DSV ± RBV
- Limitations
- Exclusion of cirrhosis
- Absence of black patients
- Low number of experienced patients with genotype 1a