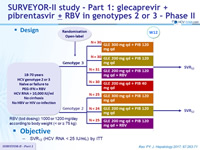
SURVEYOR-II study - Part 1: glecaprevir + pibrentasvir ± RBV in genotypes 2 or 3 – Phase II
Kwo PY. J Hepatol 2017; 67 :263-71
Anti-HCV
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Ribavirin
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Ribavirin
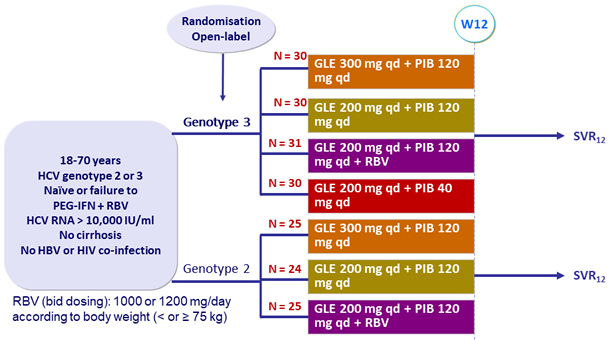
Genotype
2
3
2
3
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
No
No
Design
Objective
- SVR12 (HCV RNA < LLOQ) by ITT
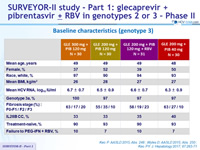
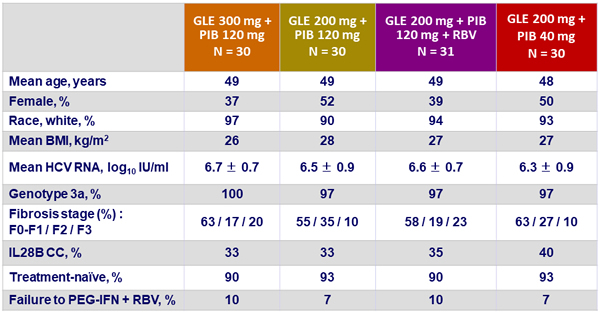
Baseline characteristics (genotype 3)
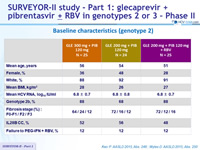
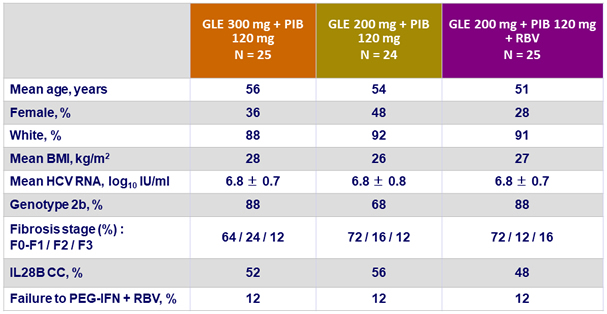
Baseline characteristics (genotype 2)
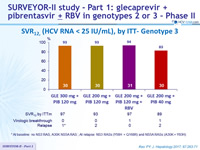
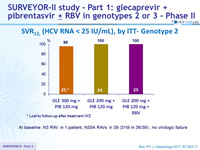
SRV12, (HCV RNA < 25 IU/mL), by ITT- Genotype 3
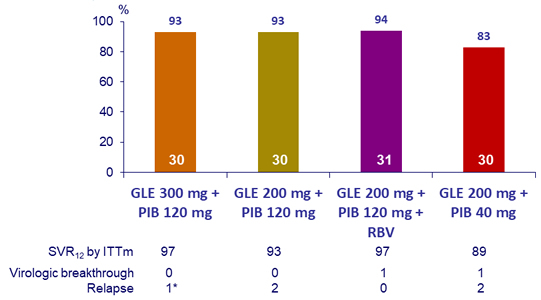
*
At baseline: no NS3 RAS, A30K NS5A RAS ; A t relapse: NS3 RASs (Y56H + Q168R) and NS5A RASs (A30K + Y93H)
SRV12, (HCV RNA < 25 IU/mL), by ITT- Genotype 2
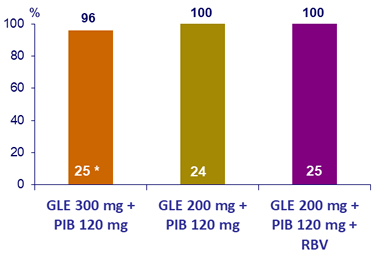
* Lost to follow-up after treatment W2
At baseline: N3 RAV in 1 patient, NS5A RAVs in 39 (31M in 36/39) ; no virologic failure
Adverse events and laboratory abnormalities (genotype 3)
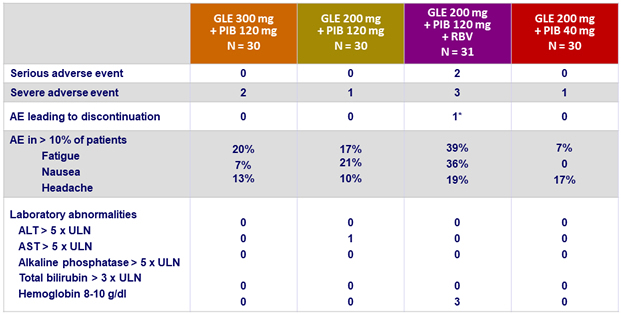
* Abdominal pain and sensation of heat
Adverse events and laboratory abnormalities (genotype 2)
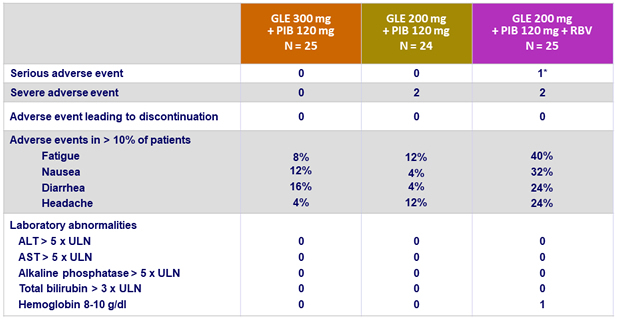
* Atrial fibrillation (not related)
Summary
- High SVR12 rates were achieved in HCV genotype 3-infected patients without cirrhosis after 12 weeks of once daily GLE
+ PIB
- All but one patient achieved SVR12 with GLE 300 mg + PIB 120 mg
- SVR12 of 100% in genotype 2 with the different regimens tested (1/75 patients lost to follow-up, no failure in the 74 others)
- All patients with baseline NS3 or NS5A RAVs achieved SVR12
- Adverse events were mostly mild in severity
- 1 discontinuation for adverse event
- Selected doses for future studies: GLE 300 mg qd + PIB 120 mg qd